This Article Changes Clinical Practice
This prospective, randomized, phase III study showed the high efficacy of abiraterone, a selective inhibitor of androgen biosynthesis, in patients with castration-refractory metastatic prostate cancer previously treated with docetaxel. The key endpoint was overall survival, which showed the substantial supremacy of abiraterone-prednisone regimen above placebo plus prednisone with a good safety profile. This is also an important confirmation of the androgen sensitivity of the tumor even in the castration-refractory state, which is probably related to the acquisition of complete tumor autonomy due to androgen synthesis within it. Also, this study redefines the castration level of testosterone to 1-2ng/dl. Trial registration: NCT00638690
Rna Extraction And Qrt
Total RNA was exacted by using RNAiso PLUS following the manufacturers instructions. Total RNA was used for synthesizing the first strand cDNA by using Revert Aid First Strand cDNA Synthesis Kit . 2×SYBR Green qPCR Master Mix were used for the qPCR. The relative mRNA expression levels were normalized to ACTIN. The statistical difference of the results was analyzed by Students t-test, and the significance of data was determined by p-value < 0.05.
In Vitro Kinase Assay
pcDNA3.1-Flag-myr-AKT1 was transfected to HEK-293T cells, and the myr-AKT1 proteins firstly were immunoprecipitated with the anti-Flag antibody. The myr-AKT1 immunoprecipitates were resuspended in 500l 1× kinase buffer including 500M ATP , and then co-cultured with 1ug recombinant proteins GST-UHRF1-WT or GST-UHRF1-T210A or negative control at 30°C for 30min, and then the reaction was stopped by adding SDS sample buffer at 95°C for 5min. The sample were used to western blotting.
Don’t Miss: Side Effects Of Lupron Injections For Prostate Cancer
Fda Grants Priority Review To Olaparib Combination For Mcrpc
Olaparib in combination with abiraterone and prednisone, or prednisolone may soon be an FDA-approved treatment for adult patients with metastatic castration-resistant prostate cancer.
The FDA granted priority review for the supplemental new drug application for olaparib in combination with abiraterone and prednisone or prednisolone for treatment of adult patients with metastatic castration-resistant prostate cancer , according to a press release from AstraZeneca.1
Olaparib is being collaboratively developed by both AstraZeneca and MSD Research Laboratories.
There remains a critical unmet need among patients diagnosed with metastatic castration-resistant prostate cancer, where the prognosis remains poor and treatment options are limited. Todays news is another step towards bringing forward a new, much-needed treatment option in this setting. If approved, Lynparza with abiraterone will become the first combination of a PARP inhibitor and a new hormonal agent for patients with this disease, said Susan Galbraith, executive vice president, Oncology Research and Development, of AstraZeneca, in a press release.
Patients were deemed ineligible if they have a known additional malignancy that has had progression or has required active treatment in the last 5 years, have myelodysplastic syndrome or acute myeloid leukemia, clinically significant cardiovascular disease, uncontrolled hypertension, or a history of uncontrolled pituitary or adrenal dysfunction.2
References:
Abiraterone Acetate In Castration
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details. |
| First Posted : March 19, 2008Results First Posted : May 16, 2013Last Update Posted : April 30, 2014 |
| Drug: PlaceboDrug: Abiraterone acetateDrug: Prednisone/prednisolone | Phase 3 |
Study medication, abiraterone acetate,is an oral medication to be administered as four 250mg abiraterone acetate tablets or 4 placebo tablets to be taken at least one hour before or two hours after a meal anytime up to 10PM everyday. Prednisone will be administered as 5mg orally twice a day for both groups. Each cycle will be 28 days. Study treatment will continue until disease progression as determined by investigator or when the patient meets criteria for withdrawal from study.
Also Check: Can You Check Your Prostate Yourself
Making Treatment Choices: Olaparib Or Rucaparib
Often, when multiple drugs are approved for the sameor in this case, a very similaruse, the side effects associated with each drug can help doctors decide which therapy is best for each patient.
Overall, Dr. Sartor explained, there arent notable differences in the types or severity of the side effects caused by olaparib and rucaparib.
And although most patients seem to handle the side effects caused by both drugs relatively well, he continued, they can cause substantial problems, including anemia, severe drops in white blood cell count, nausea, and vomiting.
Dr. Karzai also pointed to the risk of myelodysplastic syndrome, a disorder that affects the formation of blood cells in the bone marrow and that has been seen in a very small percentage of patients treated with PARP inhibitors.
These drugs definitely require close monitoring , Dr. Sartor said.
One potential advantage of rucaparib over olaparib could be the eventual availability of a blood test, called a liquid biopsy, that can identify men with BRCA1 or BRCA2 alterations who are candidates for the drug. This liquid biopsy, called FoundationOne Liquid CDx, is currently being evaluated by FDA and a decision on its approval is expected soon, according to a spokesperson for Foundation Medicine, which manufactures the test.
In addition to tissue quantity, there are also issues with the type of tissue that comes from the biopsy and its quality. There a lot of variables that can make it difficult, she added.
Pairwise Comparison In The Overall Population
Overall, median follow-up was similar: 18.27 months in the enzalutamide cohort and 19.07 months in the abiraterone cohort . Median enzalutamide treatment duration was 9.93 months versus 8.47 months with abiraterone. After adjusting for age, race, individual comorbidities, and pre-index treatments, enzalutamide-treated patients had a 16% lower risk of death versus abiraterone-treated patients . Median OS was longer in the enzalutamide cohort versus the abiraterone cohort .
Fig. 2: Adjusted KaplanMeier curve for overall survival in all patients with chemotherapy-naive mCRPC treated with either enzalutamide or abiraterone irrespective of follow-up treatments.
*Enzalutamide versus abiraterone. ABI abiraterone, ENZA enzalutamide, HR hazard ratio, IQR interquartile range, mCRPC metastatic castration-resistant prostate cancer, OS overall survival.
Read Also: Where Is The Prostate Gland On A Guy
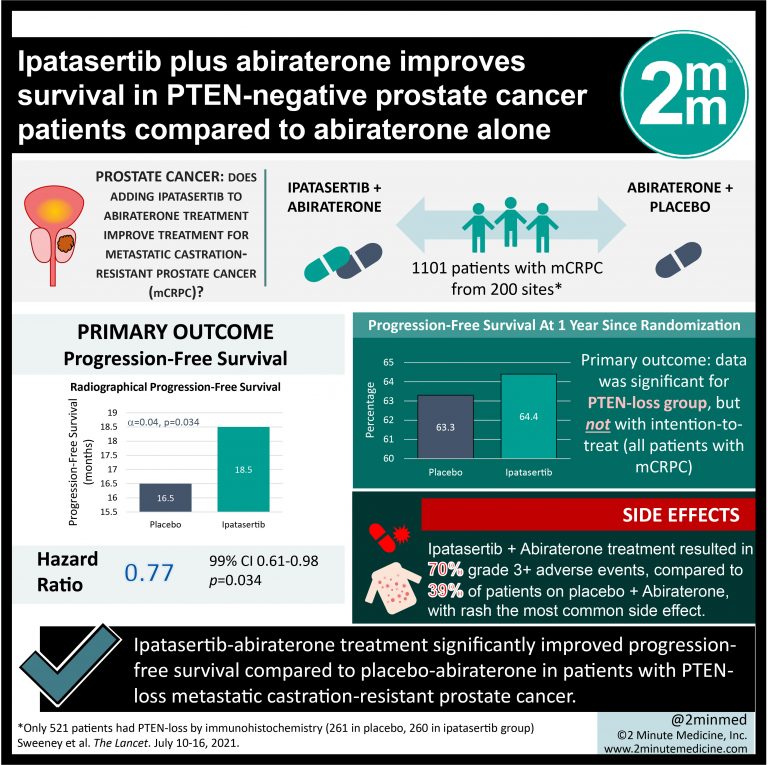
Abiraterone Improves Survival In Metastatic Prostate Cancer
A multinational phase III trial found that the drug abiraterone acetate prolonged the median survival time of patients with metastatic castration-resistant prostate cancer by 4 months compared with patients who received a placebo. The preliminary results from the study were presented October 11, 2010, at the 35th Congress of the European Society for Medical Oncology in Milan, Italy, and subsequently published in the New England Journal of Medicine on May 26, 2011 .
Standard prostate cancer treatments reduce blood levels of testosterone, the hormone that fuels the cancers growth. However, most prostate cancers eventually become resistant to these treatments. Such cancers are called castration-resistant prostate cancers. Abiraterone acetate is designed to treat these tumors by inhibiting the production of androgen in the testes, adrenal glands, and prostate cancer tumors themselves.
The clinical trial included 1,195 patients from 13 countries whose metastatic prostate cancer had previously been treated with one of two chemotherapy regimens that included docetaxel. Among the 797 patients randomly assigned to receive abiraterone acetate plus the corticosteroid prednisone, median overall survival was 14.8 months. Among the 398 who received prednisone plus placebo, median survival was 10.9 months.
- December 31, 2012
Abiraterone/adt Combo Increased Prostate Cancer Survival
Combined therapy with abiraterone acetate/prednisone plus ADT significantly improved overall survival and radiographic progression-free survival among men with metastatic hormone-naive prostate cancer.
Combined therapy with abiraterone acetate/prednisone plus androgen deprivation therapy significantly improved overall survival and radiographic progression-free survival among men with metastatic hormone-naive prostate cancer compared with ADT and placebo alone, according to the results of the phase III LATITUDE trial presented at the 2017 ASCO Annual Meeting.
In my opinion, these findings support the fact that adding abiraterone and prednisone to castration should now be considered the new standard of care for men with newly diagnosed metastatic prostate cancer, said researcher Karim Fizazi, MD, PhD, head of the department of cancer medicine at Gustave Roussy, University Paris-Sud, Villejuif, France.
Historically, ADT has been the standard of care for patients with metastatic prostate cancer. However, at approximately 1 year on ADT about one-half of patients with metastatic disease have progressed. More recently, ADT plus docetaxel has been the new standard of care.
In LATITUDE, the researchers tested adding abiraterone plus prednisone to ADT. Abiraterone was already an approved agent for castration-resistant disease, and is associated with improved overall survival in this setting.
Related Content:
Read Also: What Is Better For Prostate Cancer Surgery Or Radiation
Similar Articles Being Viewed By Others
Carousel with three slides shown at a time. Use the Previous and Next buttons to navigate three slides at a time, or the slide dot buttons at the end to jump three slides at a time.
14 September 2022
21 March 2021
H. M. Westgeest, M. C. P. Kuppen, C. A. Uyl-de Groot
14 January 2019
Edoardo Francini, Kathryn P. Gray, Christopher J. Sweeney
13 September 2018
Chang Wook Jeong, Minyong Kang, Cheol Kwak
13 November 2019
Magali Rouyer, Stéphane Oudard, on behalf of the FUJI Investigators
19 August 2020
Rana R. McKay, Rebecca Silver, Mary-Ellen Taplin
A Correction to this article was published on 15 July 2022
A Correction to this article was published on 17 January 2022
This article has been
Please Discuss The Key Findings From The Triton2 And Profound Clinical Trials Of Rucaparib And Olaparib
In May 2020, there were 2 FDA approvals for PARP inhibitors in prostate cancer within 5 days. The first was rucaparib, a PARP inhibitor approved based on a phase 2 single-arm study without a control group, called TRITON2. The TRITON2 study, in an unprecedented way, led to FDA approval of a drug in prostate cancer without requiring randomization and without having a control group. However, it was an accelerated FDA approval, meaning that full approval by the FDA is contingent upon a positive phase 3 randomized study , which is ongoing.
The second trial, which was the PROfound , were randomized to receive either olaparib or a physicians choice of hormonal therapy. The trial was positive, showing a significant prolongation of radiographic progression-free survival but also overall survival in the patients who had BRCA1, BRCA2, and ATM mutations.
As a prespecified secondary analysis, the trial was also powered to evaluate the overall patient population that included patients with mutations in any of the 15 HRR genes, not just the 3 mentioned above. In that entire study population, there was also a prolongation of radiographic progression-free survival with olaparib compared with placebo. This led the FDA to approve olaparib for not just the BRCA1, BRCA2, and ATM genes, but also various other HRR genes, including CHEK2, PALB2, CDK12, and several others.
Also Check: Can Prostate Cancer Cause Back Pain
Olaparib Tolerability And Common Adverse
- Mustafa ÃzgüroluAffiliations
- The safety profile of olaparib in men with mCRPC was similar to other solid tumours.
- Events of anaemia, nausea, decreased appetite and fatigue occurred most frequently.
- Incidences of all four events peaked within the first 2 months of starting treatment.
- All four were generally manageable through dose modifications and supportive therapies.
- Baseline bone metastases or prior taxane use was unrelated to the rate of anaemia.
Abiraterone In The Management Of Castration
- The SAGE Encyclopedia of Cancer and Society2015
- The SAGE Handbook of Healthcare2008
- The SAGE Handbook of Healthcare2008
- John E. Morley and more…Encyclopedia of Health & Aging
- The SAGE Encyclopedia of Cancer and Society2015
- The SAGE Handbook of Risk Communication2015
- Encyclopedia of Medical Decision Making2009
Don’t Miss: Is Prostate Cancer Genetically Linked
Abiraterone Acetate And Prednisolone With Or Without Enzalutamide For High
- Cancer Institute, University College London, London, UKUniversity College London Hospitals, London, UK
- Oncology Institute of Southern Switzerland, Bellinzona, SwitzerlandUniversita della Svizzera Italiana, Lugano, Switzerland
- Yeovil District Hospital NHS Foundation Trust, Yeovil, UKMusgrove Park Hospital, Taunton, UK
- A complete list of STAMPEDE investigators and collaborators is provided in the appendix Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy investigatorsFootnotes A complete list of STAMPEDE investigators and collaborators is provided in the appendix Authors List
Adverse Reactionsadjuvant Treatment Of Gbrcam Her2
Most common adverse reactions in 10% of patients who received LYNPARZA in the adjuvant setting for OlympiA were: nausea ,fatigue , anemia , vomiting , headache , diarrhea , leukopenia, neutropenia , decreased appetite , dysgeusia , dizziness , and stomatitis .
Most common laboratory abnormalities in 25% of patients who received LYNPARZA in the adjuvant setting for OlympiA were: decrease inlymphocytes , increase in mean corpuscular volume , decrease in hemoglobin , decrease inleukocytes , and decrease in absolute neutrophil count .
Read Also: What Are The Effects Of Removing The Prostate
Fda Grants Swift Review To Lynparza In First
AstraZeneca and Merck & Cos PARP inhibitor Lynparza is already used to treat prostate cancer associated with a specific genetic mutation, but could see its use broadened if a new marketing application is approved by the FDA.
The US regulator has started a priority review of Lynparza in combination with abiraterone and prednisone or prednisolone as a first-line treatment for metastatic castration-resistant prostate cancer based on the results of the PROpel trial.
The study which included an all-comer population of men with mCRPC showed that Lynparza plus Johnson & Johnsons hormonal therapy Zytiga reduced the risk of disease progression or death by 34% versus Zytiga alone, with radiographic progression-free survival of 24.8 months and 16.6 months, respectively.
Lynparza was approved in 2020 as a second-line therapy for homologous recombination repair gene-mutated mCRPC last year, but extending its label to include non-HRR patients in the frontline setting would dramatically increase the number of patients eligible for treatment.
First approved in 2014, the drug is also used for a range of indications across ovarian, breast, fallopian tube, peritoneal and pancreatic cancers, and remains one of AZs fastest growing oncology therapies, with sales rising 18% to almost $1.3 billion in the first half of this year.
Donât miss your daily pharmaphorum news.
Adverse Reactionsmaintenance Recurrent Ovariancancer
Most common adverse reactions in 20% of patients who received LYNPARZA in the maintenance setting for SOLO-2 were: nausea, fatigue ,anemia , vomiting ,nasopharyngitis/upper respiratory tract infection /influenza ,diarrhea , arthralgia/myalgia ,dysgeusia , headache , decreasedappetite , and stomatitis .
Study 19: nausea , fatigue , vomiting , diarrhea , anemia , respiratory tract infection, constipation , headache , decreased appetite , and dyspepsia.
Most common laboratory abnormalities in 25% of patients who received LYNPARZA in the maintenance setting were:increase in mean corpuscular volume , decrease in hemoglobin , decrease in leukocytes ,decrease in lymphocytes , decrease in absolute neutrophil count , increase in serum creatinine ,and decrease in platelets .
Read Also: How Far In Is The Prostate
The Levels Of Uhrf1 And P
Fig. 1: The levels of p-AKT and UHRF1 elevated in the abiraterone-refractory PCa cells.
A, B The parental or abiraterone-refractory CWR22Rv1 or LNCaP cells were treated with stepwise concentrations of abiraterone, and the IC50 values of abiraterone were calculated. C, D The protein levels of p-AKT, AKT, UHRF1, NCAM1, and SYP in the parental or abiraterone-refractory PCa cells were assessed by western blotting. E, F The expression level of UHRF1 and p-AKT were compared in the PCa tumor specimens from prostate specific PTEN/P53/Rb1 or PTEN gene knockout mice. The presented results were representative of experiments repeated at least three times. Data was represented as mean±SD. *P< 0.05, **P< 0.01, ***P< 0.001.
Treatment With Abiraterone Significantly Improves Survival In Advanced Prostate Cancer
- By Charlie Schmidt, Editor, Harvard Medical School Annual Report on Prostate Diseases
In December, researchers reported findings from a study showing that the drug abiraterone halves the risk of prostate cancer death among a specific group of patients who previously would not have been treated with it. Currently, abiraterone is approved only for men with prostate cancer that is spreading in the body. But men enrolled in the study were treated at earlier stages, before their tumors had a chance to spread. Based on the findings, the investigators concluded that abiraterone should considered for treating aggressive prostate cancer that has not yet begun to spread to other sites, but likely will in the future.
Abiraterone was first approved in 2011, specifically for metastatic prostate cancer that no longer responds to chemotherapy or drugs that block testosterone . Treatments that block testosterone production in the testicles and other glands are called androgen deprivation therapies, or ADT. Some tumors get around ADT by making their own testosterone, however, and that’s where abiraterone comes into the picture: it prevents cancer cells from making the hormone. Doctors give abiraterone together with prednisolone, a steroid that lessens treatment side effects. More recently, abiraterone’s approval was extended to men who still respond to ADT or have not yet been treated with chemotherapy.
Read Also: Best Treatment For Prostate Enlargement
Fda Grants Priority Review To Olaparib/abiraterone Regimen In Metastatic Castration
The FDA has granted priority review to a supplemental new drug application seeking the approval of olaparib in combination with abiraterone acetate and prednisone or prednisolone in adult patients with metastatic castration-resistant prostate cancer.
The FDA has granted priority review to a supplemental new drug application seeking the approval of olaparib in combination with abiraterone acetate and prednisone or prednisolone in adult patients with metastatic castration-resistant prostate cancer .1
The application is supported by findings from the phase 3 PROpel trial , in which the olaparib plus abiraterone and prednisone or prednisolone significantly prolonged imaging-based progression-free survival vs placebo plus abiraterone and prednisone or prednisolone , at 24.8 months vs 16.6 months, respectively .2 These data proved to be consistent with what was observed with blinded independent central review .
Under the Prescription Drug User Fee Act, the regulatory agency is expected to decide on the sNDA in the fourth quarter of 2022.
Stratification factors included distant metastasis type at baseline and by docetaxel treatment at the metastatic stage of disease .
Overall survival served as a key secondary end point, as well as time to first subsequent therapy or death, time to second progression or death , and health-related quality of life. Exploratory end points included objective response rate , prostate-specific antigen response rate, and time to PSA progression.
