Why Should I Travel To Germany For Lutetium
The nuclear medicine community in Germany is extremely strong. Germany was home to the first study evaluating the use of 177Lu-PSMA-617 radioligand therapy for advanced prostate cancer patients.
At the time, the multi-center study by Rahbar et al. had the largest number of patients participating at 12 centers around Germany. The study, initiated by the German Society of Nuclear Medicine, was available online in October 2016 and published in the Journal of Nuclear Medicine in January 2017,.
How Does Lutetium Psma Therapy Work
PSMA is a type of protein found on the surface of a cell. Its located on the prostate gland, some tumours, and normal tissues.
If you have prostate cancer, youll have more PSMA than normal. If the prostate cancer has spread to other parts of the body , the PSMA will also be there.
Lutetium-177 PSMA therapy uses a molecule which attaches itself to the PSMA receptors on the cancer cells.
Before its given to you, the PSMA molecule is bound with lutetium-177. This is a radioactive substance that damages and destroys the prostate cancer cells in a targeted way.
The PSMA molecule transports the lutetium-177 direct to the tumour site. That means the rest of your body isnt exposed to radiation.
Also Check: Foods That Irritate The Prostate
How Do I Know If Psma Therapy Is Right For Me
If you have been diagnosed with mCRPR, you may be eligible for 177Lu-PSMA-617 therapy. While the treatment is under review in the United States, it is available in countries like Germany. Find out if PSMA is suitable for you. Please reach out to our specialists at Qunomedical to discuss your unique case.
You May Like: Mayo Clinic Prostate Cancer Diet
The Royal Marsden To Start Offering Lutetium Psma Therapy To Treat Advanced Prostate Cancer
Patients with advanced metastatic castration resistant prostate cancer to be offered an innovative molecular therapy which precisely targets cancerous cells reducing the exposure to the rest of the body.
Lutetium-177 PSMA is an innovative therapy used to treat metastatic castration resistant prostate cancer. The therapy works to reduce the tumour size and prevents the tumour from increasing, whilst also helping to improve the symptoms that these tumours might cause.
PSMA is naturally found on the surface of prostate cells in someone with prostate cancer there is an increase of PSMA expression. If the prostate cancer has spread to other parts of the body the PSMA will also be present in those areas. When administered intravenously the Lutetium-177 PSMA ligand will travel to those areas where the PSMA is present and emits radiation that will destroy the cancer cells the treatment is targeted to the cancer with very little radiation exposure to other parts of the body.
The Royal Marsden will offer PSMA therapy, which will be administered by an expert multi-disciplinary team. Nuclear Medicine consultants, Urologist consultants, specialised Nuclear Medicine nurses, Ward nurses, Nuclear Medicine Technologists, and Nuclear Medicine Physics team will work together to ensure the best treatment and care.
All the potential side effects and the necessary precautions will be carefully explained to patients during the clinic appointment with the Nuclear Medicine consultant.
Is Lutetium 177 Psma Fda Approved
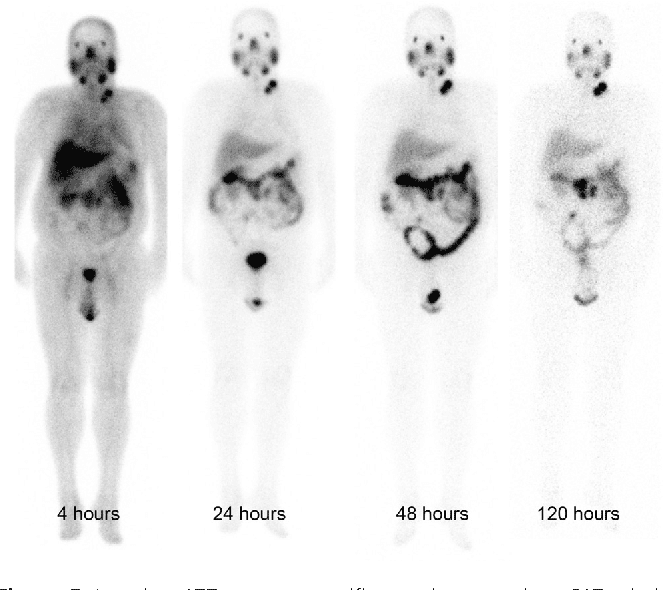
177Lu-PSMA-617 is currently under review by the US FDA. In September 2021, the FDA accepted this new drug under priority review. The decision for approval is estimated to be made in the first half of 2022.
In June 2021, the FDA granted 177Lu-PSMA-617 a Breakthrough Therapy Designation . The BTD process is designed to expedite the review of drugs intended for serious conditions where early clinical results indicate improvement over already available therapies.
Note: This BTD status for 177Lu-PSMA-617 is not to be confused with the FDAs approval of another lutetium therapy: 177Lu-Dotatate, for the treatment of pancreatic and gastrointestinal cancers. While this therapy uses Lutetium-177 to treat cancers, it does not target the PSMA proteins found on prostate cancer cells.
Read Also: What Is Focal Therapy For Prostate Cancer
Are You Searching For Possible Treatment Options For Metastatic Prostate Cancer
Prostate Cancer is the most common form of cancer diagnosed in men. While most patients respond well to surgery or radiation treatment, some develop advanced disease and become incurable. Prostate cancer is the second leading cause of cancer deaths in men.
The American Cancer Society estimates that over 30,000 new cases of prostate cancer were diagnosed in 2020 alone. Prostate cancer is usually treated by various methods including Surgery , radiation therapy, hormonal therapy, chemotherapy, immunotherapy as well as targeted therapy which has been recently added to the list. In the early stages of cancer, these methods might give long-term remission but in advanced metastatic prostate cancer, they only help in shrinking the tumors instead of curing the disease.
There are two main types of treatment that are commonly used in advanced metastatic castration-resistant prostate cancer i.e. chemotherapy and radiation therapy. Both methods not only kill the cancer cells but also affect the nearby healthy tissues and therefore, are not very effective against prostate cancer because they dont target the cancer cells only.
Recommended Reading: How To Tell Prostate Cancer
Whats The Aim Of The Trial
Were running this phase 3 trial in adult male patients with mHSPC to find out if an investigational radioligand therapy, called 177Lu-PSMA-617, is safe and effective when combined with a standard-of-care therapy, compared to the standard-of-care therapy alone. Participation in the study is voluntary. You may leave at any time or for any reason. Thank you for your interest in learning more.
Study drug are either investigational or being studied for a new use. Efficacy and safety have not been established and there is no guarantee that the study drug will become commercially available for the use under investigation.
You May Like: Radiation Therapy For Intermediate Prostate Cancer
What Is Psma Treatment
PSMA is a microscopic protein located in the prostate gland.
PSMA levels are many times higher in prostate cancer patients than in the general population. PSMA is a good target for treating advanced prostate cancer over this.
Many prostate tumors, specifically those that have progressed or grown resistant to hormone therapy, display a specific receptor termed Prostate-Specific Membrane Antigen on their cell surface .
The PSMA receptor is expressed over the locations of metastases when prostate cancer metastasizes or spreads to other regions of the body.
For various reasons, PSMA is a great deal of emphasis for both radionuclide imaging and therapy of prostate cancer:
It can be found in almost all cases of prostate cancer, at any stage of the disease.
It gets upregulated in hormone-refractory or metastatic disease
It is a cell membrane protein that is not released into the bloodstream.
After Antibody Binding, PSMA is internalized .
Mechanism of Action
A radioactive atom is conjugated to a medicinal molecule under this treatment. The medicine is then put into the bloodstream via intravenous or intramuscular, where it can directly connect to cancer cells.
The medication can detect the presence of a chemical called prostate-specific membrane antigen, which can be used to identify these cells .
What Are The Side
Overall, major side effects were minimal in those who received 177Lu-PSMA-617. They included nausea and bone marrow damage, the latter of which constrain blood transfusions in around 13% of patients.
PSMA Lu177 is well tolerated. A little dry mouth, tiredness, and physical fatigue are the most prevalent adverse effects.
About 23 weeks following the Lu177 PSMA delivery, there may be a modest drop in white blood cells and platelets as well.
These side effects are usually mild and transitory, and they go away on their own without any active therapy.
Serious or life-threatening consequences are quite unlikely.
Is PSMA PET Scan is Safe?
The radioactive molecule is extremely safe it is administered in very small doses, and hundreds of thousands of patients who have received PSMA radiotracers have reported no side effects.
After years of clinical investigation, the FDA recently approved it.
Also Check: How To Fix Enlarged Prostate Naturally
Current Clinical Evidence For The Use Of Lu Psma In Metastatic Prostate Cancer
There are currently a limited number of published trials in the use of Lu PSMA for the treatment of mCRPC .2, 3, 4, 5, 6, 8, 9, 27, 39 These are in the main small, retrospective trials, but they have shown almost uniformly is that a high proportion of men treated with 177Lu PSMA have a significant treatment response, and that bar a number of lowgrade toxicities, the treatment appears well tolerated.
What Causes Prostate Cancer
Prostate cancer is the most common form of cancer in men over the age of 50. Currently, researchers are unable to determine exactly what causes prostate cancer. DNA changes that are inherited or caused by certain lifestyle choices are believed to play a role in why some individuals develop cancer and others do not. It is believed that inherited genes account for about 5 to 10 percent of prostate cancers. Exposure to radiation, chronic inflammation, exposure to cancer-causing chemicals, high levels of androgens, or insulin-like growth factor-1 are also all believed to cause gene changes which lead to cancer.
Read Also: Bowel Problems After Radiotherapy For Prostate Cancer
Targeting Psma: Not Just For Imaging
Like a number of other radiopharmaceuticals, 177Lu-PSMA-617 has two components: a drug that delivers the therapy to cancer cells and a radioactive particle. In the case of 177Lu-PSMA-617, the delivery vehicle is PSMA-617, a drug that latches onto a protein called PSMA that is often found at high levels on the surface of prostate cancer cells. The radioactive component is lutetium-177, which is being tested as a part of multiple radiopharmaceutical drugs.
As Dr. Morris explained, PSMA-617 is extremely adept at finding and locking on to the PSMA protein on cells. Once it binds to PSMA on a cancer cell, the whole molecule is internalized by the cell and the cell is exposed to a lethal dose of radiation from lutetium-177, he said.
The PSMA protein is also at the heart of a new type of imaging procedure called PSMA PET. This form of PET imaging is just starting to be used in men with prostate cancer to determine whether their cancer has spread, or metastasized, beyond the prostate. In the last several months, FDA has approved two such drugs, known as radiotracers, for PSMA PET imaging.
Why Choose Qunomedical For Your International Medical Needs
Millions of Americans travel to other countries for their medical needs each year. There are many reasons why.
One factor is that treatment may cost less in other countries compared to the United States. This may be a more significant driving factor in recent years, as almost 5.4 million Americans lost their health insurance during the Coronavirus pandemic a record high.
Many people also seek procedures or therapies first approved in other countries before becoming available in the United States.
Planning for medical treatment abroad can be difficult. How do you know where to start? Where do you find qualified medical professionals that fit your medical needs? How do you manage hospital stays, pricing, and travel plans while you may be suffering from pain, fatigue, and other issues?
Founded by a medical professional, Qunomedical offers a concierge approach to high-quality and affordable medical treatments abroad. Every month, we connect over 6,500 patients with professionally-vetted doctors around the world. We offer 24/7 personalized support and everything you need to make an informed decision. Get your free assessment here.
Related Tags
You May Like: Refusing Treatment For Prostate Cancer
You May Like: Early Detection Of Prostate Cancer Survival Rate
What To Expect During Lu
PSMA therapy involves radioactive medicine, also known as radiopharmaceuticals. Because of this, the therapy is administered in a hospitals nuclear medicine department.
A medical professional will inject the medicine into your arm. You will have to wait about 30 minutes for the drug to spread through your bloodstream. Your doctor may also prescribe anti-nausea medication and a diuretic to help eliminate the Lu-177 from your body. You will be monitored at regular intervals and leave when your radiation levels come down.
In a few days, your doctor will schedule an imaging scan to see if the drug has targeted the right locations.
Study Aim Design And Participants
This study aims to identify routinely obtainable, and easily accessible pre- and intratherapeutic predictive factors for overall survival in a monocentric patient cohort treated with Lu-177-PSMA-617-RLT for metastasized castration resistant prostate cancer.
Overall, 56 patients suffering from mCRPC and periodically treated with Lu-177-PSMA-617-RLT at the University Hospital of Kiel, Germany, between January 2015 and December 2020 were enrolled in this observational retrospective study.
Interruption of the therapy regimen, or application of one or more therapy cycles elsewhere than in the University Hospital of Kiel, led to exclusion from the study.
Lu-177-PSMA-617-RLT was applied as individual compassionate use according to the common regimen described in the national consensus advice .
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its subsequent revisions and has been approved by the institutional review board . Written informed consent was obtained from all subjects.
Also Check: Where Does Prostate Cancer Spread
What Is The Response Rate With Lu Psma Therapy
Lu177 PSMA response rates are determined by the tumor biology of prostate cancer, the initial Gleason Score, the degree of illness, and prior therapy responses.
Lu177 PSMA therapy typically results in a drop in PSA and a shrinking of tumors in more than 70% of patients.
The mindset of Doctors while doing this therapy?
Expertise says Lu PSMA Therapy is a particularly precise nuclear medicine therapy that is used to treat metastatic prostate cancer or when other methods of treatment are no longer effective.
According to doctors, patients with advanced castration-resistant metastatic prostate cancer who have shown symptoms of tumor progression are candidates for this treatment. The progression of cancer can take several forms:
PSA levels in the bloodstream are increasing.
CT/MRI or Ga68 PSMA scans show an increase in the size or number of metastatic lesions.
Increased pain or other indications of the disease getting worse
Patients cannot get Lu177 PSMA Therapy if they have any of the following conditions:
Definitions Of Outcome Parameters
Therapy response was evaluated by PSA reduction at the time of the nadir after PSMA-RLT relative to PSA before the start of the course. The term initial PSA reduction refers to PSA decrease in percentage after the first course of PSMA-RLT, whereas total PSA reduction is the PSA decrease in percentage after the last therapy course in relation to PSA levels before the first course. Response to PSMA-RLT was defined as any PSA decrease. Disease progression was noted in case of a PSA increase 25% relative to the previous nadir PSA-level. Progression-free survival for each course spans from therapy start to following progression of disease. Total PFS refers to the time from the start of the first treatment course to progression after the last PSMA-RLT-related PSA decline during the entire time of follow-up.
Read Also: Is Stage 4 Prostate Cancer Curable
Who Is A Candidate For Pluvicto
Men with metastatic, castrate-resistant prostate carcinoma are potential candidates for Pluvicto. In other words, these are men whose prostate cancer has progressed despite prior therapies such as surgical resection, radiation therapy, androgen receptor pathway inhibition, and/or taxane-based chemotherapy.
PET/CT imaging with PSMA is required to confirm that the metastases will accumulate PSMA, and therefore respond to Pluvicto.
Guidelines may change in the future. Anyone interested is encouraged to discuss Pluvicto with their cancer care provider. Inquiries can be directed to Alex DiFonzo at 519-3456, ext. 2351, or the theranostics team at
Is This Treatment Safe
The radiation used in the Lutetium-177 is designed to only destroy the cancer cells. With theranostic, treatment becomes more personalized so that cancer and its metastasis are effectively pinpointed but the whole body is never exposed to the radiation. Only the cancer cells are irradiated and destroyed. Blood tests will be performed to makes sure that the radiation is not damaging healthy tissues. Imaging tests are also performed to ensure that the radioactive material at the tumor sites has been correctly absorbed.
Side effects of the therapy may include:
- A brief decline in production of blood cells
You May Like: Can You Still Have Sex After Prostate Removal
What Are The Phases Of A Trial
Each phase helps answer different questions. An investigational drug must pass through every phase before it can be submitted for approval. 1,126 people will take part in our phase 3 clinical trial.
20-80 healthy volunteers take part in the first stage of a clinical trial. The aim is to test the safety with a very low dosage of the investigational drug.
100-300 people take part in the second phase of a clinical trial. At this stage, it might be used with a placebo to test if its an effective treatment and to learn about possible side effects.
Up to 3000 people take part in this later phase of a clinical trial. This is one of the final stages of getting a drug into market. The aim is to understand more about the investigational drug and see how it compares to existing treatments.
These trials help us learn how the treatment affects people in the long-term. They only take place once a drug has been approved for general use.
An Update To The Pilot Study Of 177lu
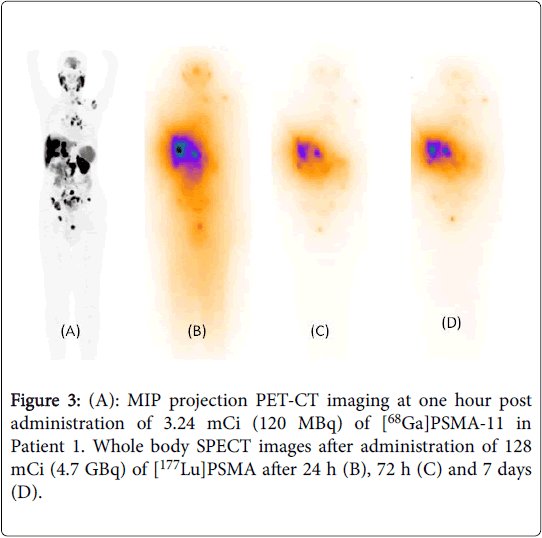
- 1Department of Radiology and Nuclear Medicine, Radboudumc, Nijmegen, Netherlands
- 2Department of Urology, Radboudumc, Nijmegen, Netherlands
- 3Department of Medical Oncology, Radboudumc, Nijmegen, Netherlands
177Lu-PSMA-617 radioligand therapy is a novel treatment for end-stage prostate cancer, which could also be applied to patients with hormone-sensitive prostate cancer with high expression levels of prostate-specific membrane antigen . In this perspective, we review the recent results of toxicity, radiation doses, and treatment effect of 177Lu-PSMA in patients with low volume metastatic hormone-sensitive prostate cancer. Moreover, we present long-term follow-up data, such as toxicity and time without androgen deprivation therapy , of the patients who participated in this trial. Overall, 177Lu-PSMA appeared to be a feasible and safe treatment modality in this setting, as well as in long-term follow-up. We observed that men with a prostate-specific antigen response of more than 50% seemed to especially benefit from this therapy by postponing ADT and thus preserving the quality of life.
Also Check: State Of The Art Prostate Cancer Treatment
