A Trial Looking At Hormone Therapy With Other Treatments For Prostate Cancer
Cancer type:
Phase:
This trial is comparing hormone therapy alone with a combination of hormone therapy and one or more other treatments for prostate cancer. Cancer Research UK supports this trial.
Please note, some parts of the STAMPEDE trial have results. We have a separate summary of the STAMPEDE trial results. These results have changed the way prostate cancer is treated. There are now more options for treatment when a man is first diagnosed with high risk prostate cancer.
Also Check: 1 Cm Lesion On Prostate
About The Stampede Trial
STAMPEDE is a large clinical trial that is assessing new treatments for men with high-risk prostate cancer. The trial has been open since 2005 and some results have been released, including observations related to patients with limited metastatic prostate cancer. Each new or alternative treatment is compared with the current standard approach, referred to as a comparison. More than 10,000 people have joined STAMPEDE so far more findings will become available as the trial continues.
Recommended Reading: How To Stimulate Prostate Gland
Innovation In Trial Design
Within the group, the researchers developed a plan to implement an innovative trial design it was a multi-arm, multi-stage design that would allow them to test a greater number of treatments faster and more efficiently. The STAMPEDE trial would have only one control arm, against which all other treatments could be tested, and there were analysis periods built in so that insufficiently active treatments could be stopped rather than continued to the end of the trial.
Table: Treatment comparisons investigated at times across the STAMPEDE trial
| Treatment |
| Standard of care plus zoledronic acid |
| Standard of care plus docetaxel chemotherapy |
| Standard of care plus celecoxib |
| Standard of care plus zoledronic acid + docetaxel |
| Standard of care plus zoledronic acid + celecoxib |
| Standard of care plus abiraterone |
| Standard of care plus abiraterone + enzalutamide |
| Standard of care plus the provision of prostate radiotherapy for men with metastases |
| Standard of care plus metformin |
| Standard of care plus transdermal oestradiol |
*Standard of care was long-term hormone therapy, plus radiotherapy if possible, in non-metastatic patients. The standard has been updated over time to include docetaxel or abiraterone, and radiotherapy across all patients
Don’t Miss: How Long Can You Live With Prostate Cancer
Is There A Rationale For Genetic Workups Before Treatment
Panellists answered this question from the floor, with Tombal explaining that current guidelines advise genetic workup in patients with a family history of cancer, with newly-diagnosed metastatic cancer, and in patients with intraductal and cribriform prostate cancer.
Patients with newly-diagnosed metastatic cancer found to have certain genetic mutations could qualify for treatment with a class of drugs called poly polymerase inhibitors that cause cell death. Otherwise, genetic workups are more for the purposes of genetic counselling, and have broader implications for research, explained Tombal.
What Is This Study About
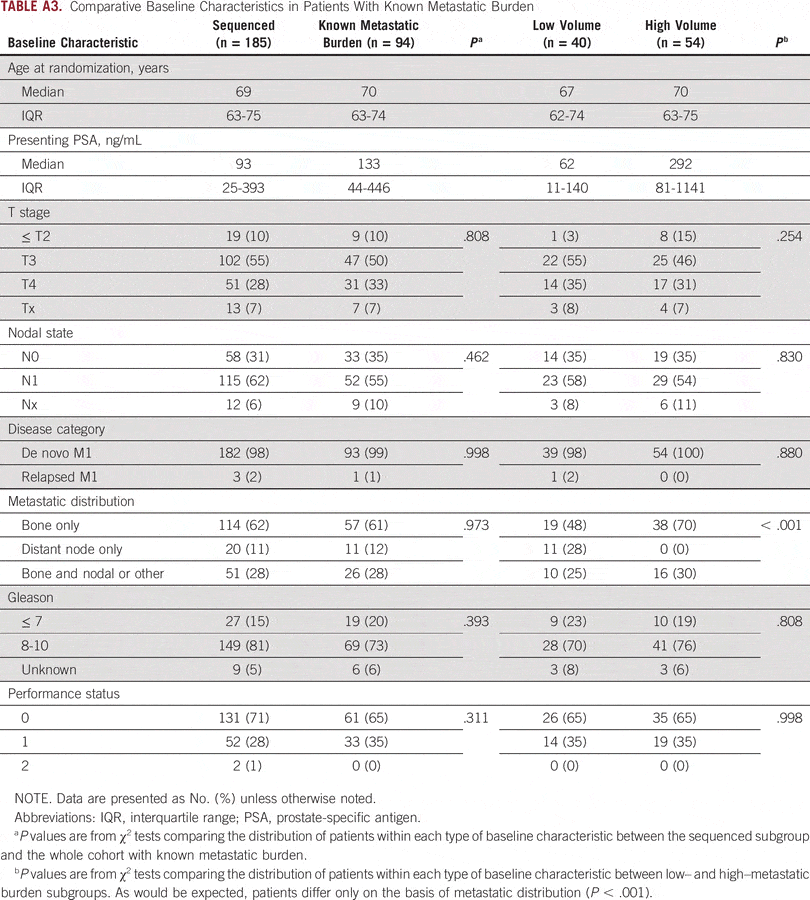
Prostate cancer accounts for around one fifth of all cancers among men. In the UK there are around 25,000 new cases of prostate cancer each year, and around 10,000 deaths.
Most men are given hormone therapy if their prostate cancer has spread , or if the cancer is very likely to spread. This usually stops the tumour from growing for a while. But in most cases over time the tumour will start to grow again.
The aim of this trial is to try to prevent the tumour re-growth by adding other treatment to the hormone therapy. The trial is currently using enzalutamide and abiraterone in combination with hormone therapy or, for newly diagnosed metastatic patients only, radiotherapy in combination with hormone therapy. Celecoxib, zoledronic acid, docetaxel and abiraterone alone have previously been tested.
Results so far
STAMPEDE âM1 Radiotherapy Comparisonâ Results: Summary:
STAMPEDE âM1 Radiotherapy Comparisonâ Results:
STAMPEDE results on docetaxel and zoledronic acid: summary for health workers
The STAMPEDE prostate cancer trial: docetaxel and zoledronic acid results for patients
Read Also: Is Ejaculation Good For Prostate
Triple Treatment Options For Metastatic Hormone
The panel then discussed options for triple therapy treatment of patients with mHSPC. High-volume mHSPC patients are considered good candidates for this approach, whereas low-volume, recurrent patients are not. The choice of ARPI to use within the triple therapy regimen should be guided by currently available evidence, which points, said Bögemann, towards darolutamide and abiraterone. Gratzke added: Even if this was not an industry-sponsored symposium, I would not know why I wouldnt be prescribing darolutamide in that aspect.
Prioritizing Qol Surveys In Clinical Trials Gives Insights Into Qol Impacts Of Life
This Reading Room is a collaboration between MedPage Today® and:
The treatment landscape for men with metastatic hormone-sensitive prostate cancer has changed dramatically over the last 5 years, with abiraterone and a number of new therapies used in combination with androgen-deprivation therapy being approved.
Data from the STAMPEDE trial and others have demonstrated that treatment with docetaxel or abiraterone started concurrently with long-term ADT prolongs survival when compared with ADT alone. As a result, patient-reported quality of life between these treatments may guide treatment choice, and the impact of additional treatment with docetaxel or abiraterone on QOL has not been directly compared.
The STAMPEDE trial is a phase III multiarm, multistage platform trial assessing different treatment regimes given alone or in combination with long-term ADT. In a study recently published in the Journal of Clinical Oncology, Rush and colleagues assessed QOL in 515 men enrolled in STAMPEDE randomized to receive docetaxel or abiraterone concurrently with ADT over a 2-year follow-up period. QOL was measured using the EORTC QLQ-C30 questionnaire with the prostate cancer-specific PR25 module, and the primary outcome was a difference in QOL with a pre-defined criterion for a clinically meaningful difference of > 4 points.
Read the study here and an interview about it here.
Primary Source
Bradley C. Carthon1, Emmanuel S. Antonarakis2
Correspondence to:
doi: 10.21037/tcr.2016.09.08
Don’t Miss: Complications Of Radiation For Prostate Cancer
Stampede: Docetaxel For Hormone
Transcript:
Dan George, MD: I want to talk to Chris because hes got a little skin in this game and has been thinking a lot about this subject. Chris, give us a little background from the clinical trial perspective. Bertrand told us a little about the natural history, biology, and prognosis. What were the data presented at ESMO , and what else should we be thinking about in terms of this debate, if you will?
Christopher Sweeney, MBBS: Thanks, Dan, for the opportunity to actually go into this. Im going to take a little time. But actually, what Ill do is try and address a few issues that have caused some confusion and try to put it all in context. Im not going to answer any 1 specific question in isolation. Im going to try to put it all into context. Lets be very clear: there has been a lot of confusion out there. People have come back to me saying, Im so confused about this. Lets explain where the confusion comes from.
Right now, where we are in 2019 in the data that got presented today at this meeting at ESMO 2019, is 2-fold. One is the updates on the N0 state, which is both a rise in PSA postprostatectomy, like the EMBARK trial. Then for the adjuvant state, like the bolus study, 82 radiation plus or minus docetaxel. Our colleague, Joe OSullivan, has been an active member in that STAMPEDE team, so weve got the whole perspective here represented.
Dan George, MD: Across studies, yes.
Transcript Edited for Clarity
Hormone Therapy Plus Docetaxel
A total of 592 men were accrued to the ADT + docetaxel arm of STAMPEDE, and 550 were eventually included in the safety analyses . Men started docetaxel a median of 2 weeks after randomization and approximately 9 weeks after beginning ADT, a timeframe similar to other trials examining ADT plus docetaxel in the context of newly-diagnosed M1 disease . About 77% of patients in this arm received all six planned cycles of docetaxel. Addition of docetaxel to ADT led to a 22% reduction in risk of all-cause death . Moreover, there was a median OS benefit of approximately 10 months over ADT alone with the addition of docetaxel. There was also a prostate cancer-specific survival benefit and a FFS benefit for the addition of docetaxel to ADT . However, while the benefit from the addition of docetaxel was noted for multiple subgroups including those with M1 disease, the benefit in men with non-metastatic disease was less clear . The M0 patients made up a smaller proportion of the enrolled patients , had less deaths at the last follow-up, and the study was probably underpowered to determine the benefit of docetaxel in this group.
Donât Miss: Cancer Returns After Prostate Removal
Recommended Reading: How To Be Tested For Prostate Cancer
Imaging And The Treatment Of Non
Tombal began the panel discussion by asking about the role of modern imaging in treatment decisions for patients with nmCRPC.
Gratzke and Bögemann agreed that a negative prostate-specific membrane antigen PET scan is not a reason to delay ARi treatment in a patient with nmCRPC who is in good shape, but shows a PSADT of 7 months.
Why would you wait if you had a drug that is able to prolong survival and metastasis-free survival with a good quality of life? said Gratzke. There is no real, good argument to do so.
A PSADT of 7 months means there is a high risk of developing metastasis within a short period of time, added Bögemann. So, even if the PSMA PET scan is negative, why wait, he agreed.
Tombal provided another example of a patient who had undergone prostatectomy and radiotherapy following rapid progression. Initially, their PSMA PET scan was negative and so ARi treatment was deferred. However, 3 months later, their PSA had doubled and a subsequent PSMA PET scan showed a large metastasis on the hip. The patients doctors opted for radiation therapy only. Tombal asked if the panel agreed with this approach.
When there is systemic disease, there is a very good rationale for offering systemic treatment, Gatzke responded. Radiation is still important, however, especially if the patient is experiencing pain or has a risk of fracture.
You May Like: Prostate Over The Counter Drugs
Hormone Therapy Plus Docetaxel And Zoledronic Acid
The STAMPEDE authors also present data on the ADT + zoledronic acid + docetaxel arm, where prespecified analysis points showed survival benefit with docetaxel. Of note, patients in this arm received greater exposure to zoledronic acid vs. patients receiving zoledronic acid alone . Even with this increased exposure, there was no effect of zoledronic acid on FFS, OS, or even SREs in patients that received this bone-targeting agent in addition to docetaxel and ADT. Most, if not all, of the benefit in this combinatorial group was derived from the docetaxel itself . FFS in the ADT + zoledronic acid + docetaxel group was improved compared to ADT alone . OS in the ADT + zoledronic acid + docetaxel group was 76 months, slightly less than those men that received ADT + docetaxel alone , raising the question of a possible negative effect of zoledronic acid to docetaxel, although this is purely speculative.
Read Also: What Does The Prostate Do In The Male Body
Stampede Results Bar Graph Showing Percentage Of Men Living At 6 Years
Side effectsMain conclusions of the STAMPEDE trial so far
- abiraterone which also reduces the chances of the cancer spreading or coming back
- radiotherapy in men with less prostate cancer spread
- abiraterone and prednisolone for men whose cancer hadnt spread to another part of the body
- zoledronic acid and adding it to docetaxel didnt add any extra benefit to having docetaxel and hormone therapy
- adding enzalutamide to abiraterone
Further results
Abiraterone Acetate And Prednisolone With Or Without Enzalutamide For High
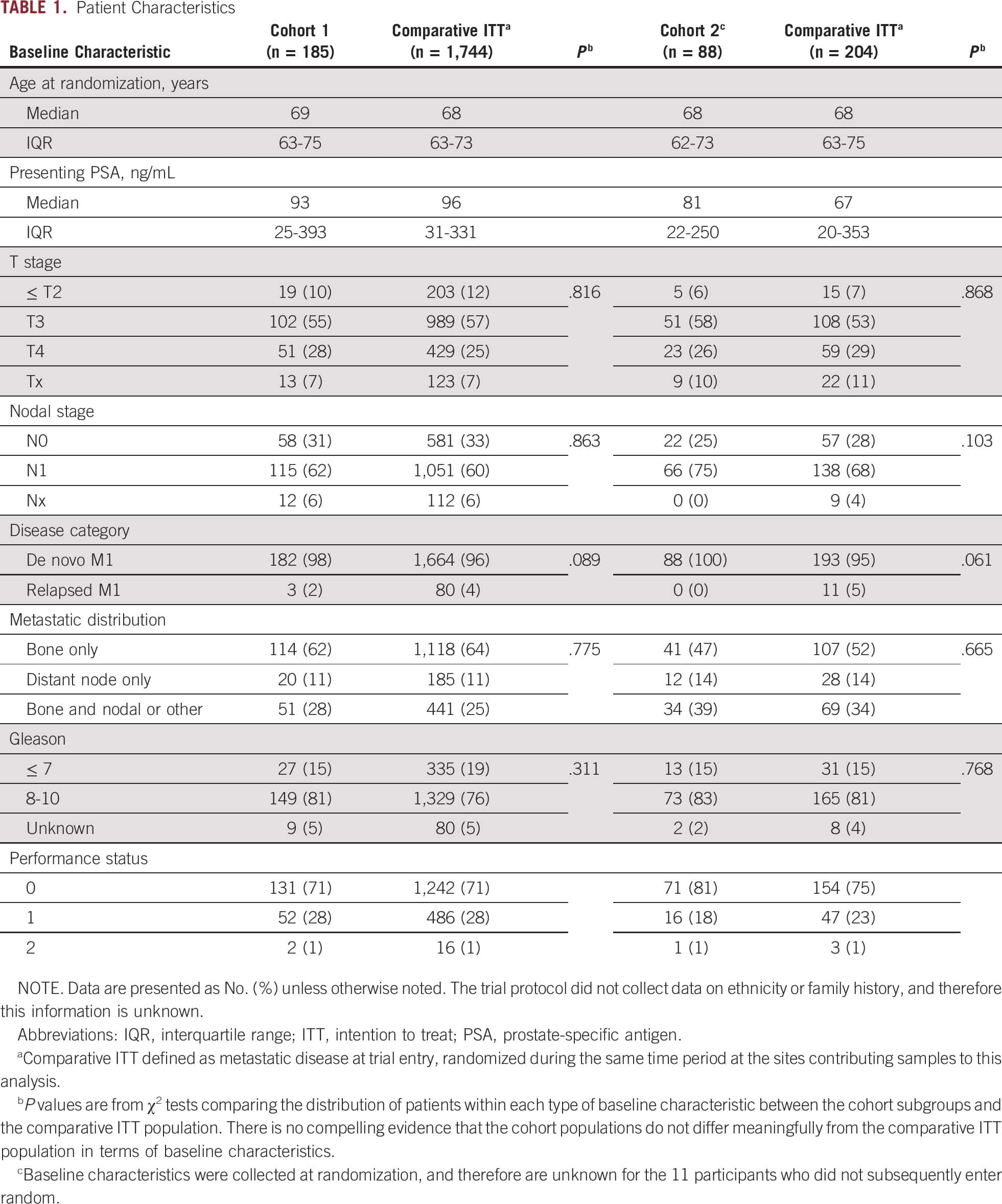
- Cancer Institute, University College London, London, UKUniversity College London Hospitals, London, UK
- Oncology Institute of Southern Switzerland, Bellinzona, SwitzerlandUniversita della Svizzera Italiana, Lugano, Switzerland
- Yeovil District Hospital NHS Foundation Trust, Yeovil, UKMusgrove Park Hospital, Taunton, UK
- A complete list of STAMPEDE investigators and collaborators is provided in the appendix Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy investigatorsFootnotes A complete list of STAMPEDE investigators and collaborators is provided in the appendix Authors List
Read Also: What Is A Prostate Test
The Problem With Standard Trial Development
Many therapies have been in development for the treatment of recurrent/metastatic prostate cancer, but the approval process has been slow . This delay in approvals can lead to high cost of drug development and clinical trials required to ensure safety and efficacy. Moreover, the most commonly used trial process, whereby positive phase II data lead to phase III trial development, is not always predictive of ultimate success in phase III. There are several examples of encouraging phase II results that did not translate to anticipated positive phase III results in prostate cancer trials , including data from combining docetaxel with the agents dasatinib , lenalidomide , calcitriol , bevacizumab and more recently using cabozantinib monotherapy .
There are several possible ways to overcome these hurdles, such that resources are not spent on large ineffective trials. One way has been to design randomized phase II trials with the power to detect more-than-modest effects. Investigators have also been more aggressive with presentation of phase II data for regulatory approval. Lastly, groups have foregone phase II trials and moved to phase III trials based on early phase I data, but this may add to the risk of a negative phase III trial. Despite these strategies, there is general agreement that the multitude of large-scale trials needed to test various agents and combinations has been time-consuming, inefficient and cost-prohibitive.
How Could The Stampede Trial Shape Prostate Cancer Care
In this video interview, filmed at the ESMO 2022 Congress , we speak with Marc Stapley CEO of Veracyte and Elai Davicioni Ph.D. Urology Medical Director of Veracyte about exciting developments in genomic tests. We also explore the STAMPEDE trial, which aims to provide evidence as to what is the best way of treating men with newly diagnosed advanced prostate cancer.
The opinions expressed in this interview are those of the author and do not necessarily reflect the views of Oncology Central or Future Science Group.
You May Like: Psa Grading Scale For Prostate Cancer
The Stampede Trial: Paradigm
Bradley C. Carthon1, Emmanuel S. Antonarakis2
1 Johns Hopkins Sidney Kimmel Comprehensive Cancer Center , , USA
Correspondence to:
Keywords: Androgen deprivation docetaxel stampede zoledronic acid
Submitted Jul 22, 2016. Accepted for publication Aug 02, 2016.
doi: 10.21037/tcr.2016.09.08
Lack Of Treatments And Evidence
As recently as ten years ago, despite many new drugs being approved for use in prostate cancer, many men with high-risk disease still had insufficient treatment options and it was unclear how well drugs work in this population. Prior to the STAMPEDE trial the standard of care for prostate cancer had not changed for many decades.
To address this challenge to the treatment of high-risk prostate cancer, researchers from MRC Clinical Trials Unit at UCL brought an idea to test a variety of current treatments in men with high-risk prostate cancer to the NCRI Prostate Group.
- a hormone patch called transdermal oestradiol
Recommended Reading: Why Would An Enlarged Prostate Interfere With Urination
Upfront Abiraterone Improves Survival For Men With Prostate Cancer
Giving the drug abiraterone alongside standard hormone therapy improves the survival of men with high-risk or advanced prostate cancer, according to results from the STAMPEDE trial, published in the New England Journal of Medicine on 3 June 2017.
In the abiraterone comparison in STAMPEDE, 957 men who were randomised to receive standard of care were compared to 960 men who were randomised to receive abiraterone plus prednisolone plus standard of care . Men in the abiraterone group had four abiraterone tablets and one prednisolone tablet a day.
Abiraterone is a type of hormone therapy that works in a different way to standard hormone therapy. Abiraterone is currently licensed to treat prostate cancer that has spread and has stopped responding to standard hormone therapy. STAMPEDE tested using it earlier, when men were starting standard hormone therapy.
The data were presented the same day at the American Society of Clinical Oncology conference in Chicago, USA.
All the men taking part in the abiraterone comparison of STAMPEDE:
- had high risk prostate cancer, or prostate cancer that has already spread to the nodes or other parts of the body
- were starting long-term hormone therapy for the first time
- were fit enough to have chemotherapy.
Abiraterone also lowered the relative chance of treatment failure by 71% compared to standard therapy. The effects were consistent across the different subgroups of men joining the trial.
The NEJM paper
Hormone Therapy Plus Zoledronic Acid
Zoledronic acid was FDA-approved in 2002 in an attempt to decrease morbidity from bone metastases in bone-tropic diseases including prostate cancer. In STAMPEDE, 593 patients were randomized to this arm between October 2005 and March 2013. Zoledronic acid was administered over six 3-weekly cycles, then every 4 weeks for up to 2 years. Preliminary analyses for FFS at predetermined intervals failed to show an effect from zoledronic acid added to standard ADT, irrespective of docetaxel use . In addition, the OS hazard ratio was 0.94 with no obvious benefit from Zoledronic acid in any of the subgroup analyses including those with metastatic disease. There also appeared to be no beneficial effect on skeletal-related events .
The role of zoledronic acid or other bisphosphonates in the context of advanced prostate cancer has been examined in several other trials and in an accompanying meta-analysis published at same time of the results noted above . The results of STAMPEDE mirrored those of the earlier findings from CALGB-90202, which was also stopped early due to lack of an effect . In that study, zoledronic acid provided no OS benefit and no improvement in SREs when added to ADT in men with bone-metastatic hormone-sensitive prostate cancer.
You May Like: How Long Does It Take For Prostate Cancer To Spread
