New Perspective On Low
The paradigms of prostate cancer detection are changing so that detection of low-risk disease is not followed, and surveillance rather than treatment is offered to those with lower-risk disease, Dr Carroll said. This sentiment was echoed by the chair of the NCCN Prostate Cancer Guideline Committee, James L. Mohler, MD, Associate Director, Translational Research, Roswell Park Comprehensive Cancer Center, Buffalo, NY, who discussed management approaches in prostate cancer.
An alternative to performing biopsies in patients with elevated prostate-specific antigen levels is the use of serum- or urine-based biomarkers that increase the specificity of screening.
What theyre doing is determining which men with an elevated PSA are harboring clinically significant disease, defined by an elevated Gleason score, said Dr Carroll. These tests miss few high-risk cancers, but decrease the biopsy rate by 30% to 40%, he added.
The other big marker right now is multiparametric MRI , Dr Carroll told attendees. Using multiparametric MRI or biomarkers misses only approximately 1% to 2% of high-risk tumors, and even fewer if both tests are used, while avoiding unnecessary biopsies and detecting fewer lower-risk cancers.
In my opinion, very few men with low-risk disease should ever be treated, Dr Carroll posited. Several studies have shown that there is no harm in delaying treatment by up to 2 years, he noted.
Agents Related To Bone Health In Crpc
In a multicenter study, 643 men with CRPC and asymptomatic or minimally symptomatic bone metastases were randomized to intravenous zoledronic acid every 3 weeks or placebo.228 At 15 months, fewer men in the zoledronic acid 4-mg group than men in the placebo group had SREs . An update at 24 months also revealed an increase in the median time to first SRE .229 No significant differences were found in OS. Other bisphosphonates have not been shown to be effective for prevention of disease-related skeletal complications. Earlier use of zoledronic acid in men with castration-naïve prostate cancer and bone metastases is not associated with lower risk for SREs, and in general should not be used for SRE prevention until the development of metastatic CRPC.230
The randomized TRAPEZE trial used a 2×2 factorial design to compare clinical PFS as the primary outcome in 757 men with bone metastatic CRPC treated with docetaxel alone or with zoledronic acid, 89Sr, or both.231 The bone-directed therapies had no statistically significant effect on the primary outcome or on OS in unadjusted analysis. However, adjusted analysis revealed a small effect for 89Sr on clinical PFS . For secondary outcomes, zoledronic acid improved the SRE-free interval and decreased the total SREs compared with docetaxel alone.
American Urological Association Recommendations
American Urological Association guidelines for the management of CRPC describe six index-patient scenarios for which recommendations could be formulated.
Index patient no. 1: Asymptomatic non-metastatic CRPC
Recommendations are as follows:
-
Observation with continued ADT
-
First-generation antiandrogens or first-generation androgen-synthesis inhibitors to patients unwilling to accept observation.
-
Systemic chemotherapy or immunotherapy should not be offered to patients with non-metastatic CRPC outside the context of a clinical trial
Index patient no. 2: Asymptomatic or minimally-symptomatic, metastatic CRPC with good performance status and without prior docetaxel chemotherapy
Recommendations are as follows:
-
Abiraterone plus prednisone, enzalutamide, docetaxel, or sipuleucel-T
-
First-generation antiandrogen therapy or ketoconazole plus steroid or observation to patients who do not want or cannot have one of the standard therapies
Index patient no. 3: Symptomatic, metastatic CRPC with good performance status and no prior docetaxel chemotherapy
Recommendations are as follows:
-
Abiraterone plus prednisone, enzalutamide, or docetaxel
-
Ketoconazole plus steroid, mitoxantrone, or radionuclide therapy for patients who do not want or cannot have one of the standard therapies
-
Radium-223 to patients with symptoms from bony metastases and without known visceral disease
-
Treatment with either estramustine or sipuleucel-T should not be offered
Recommendations are as follows:
You May Like: Effects Of Prostate Radiation Therapy
Intermittent Or Continuous Adt
In patients with castration-naïve prostate cancer, complete androgen blockade does not work, Dr Mohler said. Intermittent ADT is as good as continuous ADT in terms of overall survival , with a significant quality-of-life benefit. Intermittent ADT can be personalized based on the PSA response, Dr Mohler said. A PSA level < 0.2 ng/mL signals an outstanding response to ADT and a patient who will do well without ADT for a prolonged period.
For younger and healthier men with metastatic, castration-naïve prostate cancer, survival is improved with the addition of docetaxel to ADT. Abiraterone is a less toxic alternative to chemotherapy, with a similar effect on survival.
This has produced a change in the NCCN guideline, where we make more options available for castration-naïve disease, Dr Mohler said.
If the patient is asymptomatic, I always use intermittent ADT. I discuss a possible decrease in OS, but the trade-off is improved quality of life during off cycles, he added. Continuous ADT can be considered for men with symptomatic, castration-naïve disease, unless the PSA response is outstanding, in which case intermittent ADT is preferred.
Levels Of Evidence For Radiation Therapy Recommendations In The National Comprehensive Cancer Network Clinical Guidelines
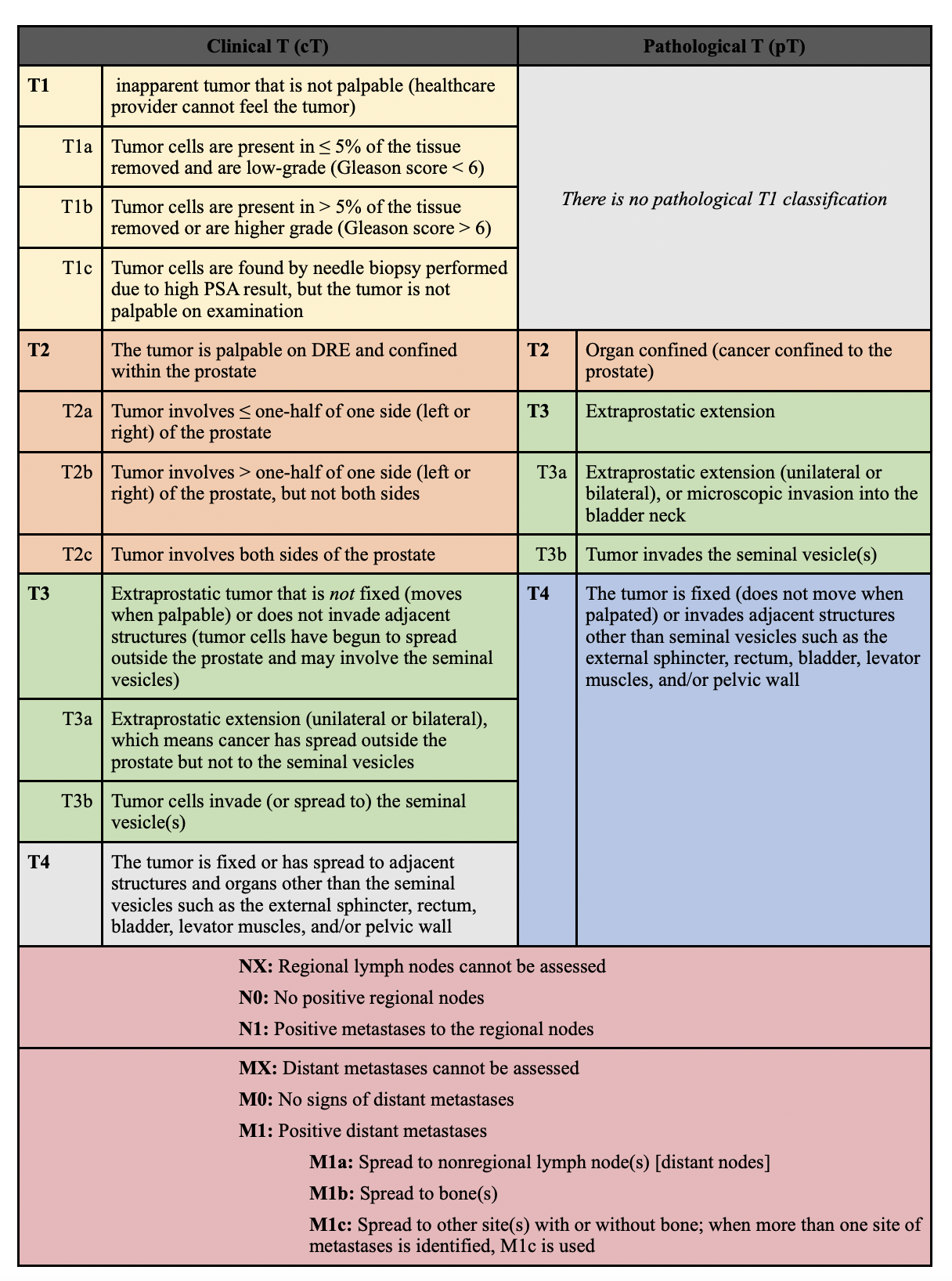
- Benjamin J. RichAffiliationsDepartment of Radiation Oncology, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, Florida
- Ricardo LlorenteAffiliationsDepartment of Radiation Oncology, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, Florida
- Deukwoo KwonAffiliationsDivision of Biostatistics, Department of Public Health Sciences, Sylvester Biostatistics and Bioinformatics Shared Resource, University of Miami Leonard M. Miller School of Medicine, Miami, Florida
- Matthew AbramowitzAffiliationsDepartment of Radiation Oncology, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, Florida
- Brandon MahalAffiliationsDepartment of Radiation Oncology, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, Florida
- Eric A. MellonAffiliationsDepartment of Radiation Oncology, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, Florida
- Department of Radiation Oncology, Penn State Cancer Center, Hershey, PennsylvaniaDepartment of Public Health Sciences, Penn State College of Medicine, Hershey, Pennsylvania
- AffiliationsDepartment of Radiation Oncology, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, Florida
Read Also: Is A Hard Prostate Always Cancer
National Comprehensive Cancer Network Recommendations
The NCCN guidelines for prostate cancer include treatment recommendations for CRPC based on the presence or absence of visceral metastases. For the most part, these recommendations are based on high-level evidence and are supported by uniform NCCN consensus .
CRPC without distant metastasis
-
Enrollment in clinical trial is preferred
-
Observation is acceptable
-
Secondary hormone therapy can be considered for patients with prostate-specific antigen doubling < 10 months anti-androgen therapy is acceptable for patients who previously received medical or surgical castration, ketoconazole, corticosteroids, diethylstilbestrol or other estrogens
CRPC with bone metastases
Measures to promote bone health include the following:
-
Zoledronic acid or denosumab
-
Avoidance of invasive dental surgery during treatment
-
Calcium and vitamin D supplements to prevent hypocalcemia during treatment
Radium-233 can be used to treat symptomatic bone metastases without visceral metastases.
Metastatic CRPC with no visceral metastases
-
Sipuleucel-T for asymptomatic or minimally symptomatic patients
-
Abiraterone plus prednisone or enzalutamide for asymptomatic patients
-
Docetaxel with prednisone for symptomatic patients may also be considered in a symptomatic patients with signs of rapid progression
-
Radium-233 for symptomatic patients
-
Secondary hormone therapy or enrollment in clinical trial may be considered
Metastatic CRPC with visceral metastases
-
Other secondary hormone therapy
-
Best supportive care
Evidence For Mpmri And Tumor Multigene Molecular Testing
The role of mpMRI in the diagnosis of prostate cancer has become increasingly important in recent years, as reflected in the NCCN and other prostate cancer guidelines.4 mpMRI typically includes diffusion-weighted imaging and/or dynamic contrast-enhanced images in addition to the standard anatomical T2-weighted imaging. The quality mpMRI involves a 3 T magnet. It has a higher signal to noise ratio, allowing quality imaging within a short time and without the use of an endorectal coil .
The NCCN guideline recommendations are based on evidence reviewed and voted on by the guideline panels. This evidence is eventually published in the Discussion section of the guidelines. However, because the NCCN guideline on prostate cancer is updated so frequently, the Discussion section often lags behind the recommendations within the same guideline. In Version 2.2022 , for example, the Discussion section is dated November 17, 2020. Many of the recent updates related to the implementation of mpMRI have been based on its increased availability and ability to stage and characterize prostate cancer.
The NCCN recommendation to use tumor molecular testing is based on the goal of achieving personalized or precision medicine. Molecular testing of a tumor offers the potential to evaluate the biologic behavior of a cancer, which would aid in clinical decision making, the guideline says.
References
Don’t Miss: Benign Prostatic Hyperplasia Icd 10 Code
Treatment By Cancer Type
NCCN MAKES NO REPRESENTATIONS OR WARRANTIES CONCERNING THE NCCN CONTENT, THE NCCN GUIDELINES OR DERIVATIVE RESOURCES PROVIDED BY NCCN, ALL OF WHICH ARE PROVIDED “AS IS.” NCCN DISCLAIMS ALL WARRANTIES, EXPRESS OR IMPLIED, INCLUDING, WITHOUT LIMITATION, THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. NCCN DOES NOT WARRANT THE ACCURACY, APPROPRIATENESS, APPLICABILITY OR COMPLETENESS OF THE NCCN CONTENT, THE NCCN GUIDELINES OR ANY DERIVATIVE RESOURCES, NOR DOES NCCN MAKE ANY REPRESENTATIONS REGARDING THE USE OR RESULTS OF THE USE OF THE NCCN CONTENT, THE NCCN GUIDELINES OR ANY SUCH DERIVATIVE RESOURCES.
NCCN EXPLICITLY DISCLAIMS THE APPROPRIATENESS OR APPLICABILITY OF THE NCCN CONTENT, THE NCCN GUIDELINES, AND ANY DERIVATIVE RESOURCES, OR THE USE OR APPLICATION OF THE NCCN CONTENT, THE NCCN GUIDELINES OR ANY SUCH DERIVATIVE RESOURCES, TO ANY SPECIFIC PATIENT’S CARE OR TREATMENT. ANY CLINICIAN SEEKING TO APPLY OR CONSULT THE NCCN CONTENT, THE NCCN GUIDELINES AND/OR ANY DERIVATIVE RESOURCES IS EXPECTED TO USE INDEPENDENT MEDICAL JUDGMENT IN THE CONTEXT OF THE INDIVIDUAL CLINICAL CIRCUMSTANCES TO DETERMINE ANY PATIENT’S CARE OR TREATMENT.
Rising Psa After Radiotherapy And Negative Conventional Imaging
For men for whom salvage local or regional therapy is not planned or is inappropriate, there is little evidence that NGI will alter treatment or prognosis. The role of NGI in this scenario is unclear and it should not be offered, except in the context of an institutional review boardapproved clinical trial.
For men for whom salvage local or regional therapy is contemplated, evidence supports NGI for detection of local and/or distant sites of disease. Findings on NGI could guide management in this setting . PSMA imaging , 11C-choline or 18F-fluciclovine PET/CT or PET/MRI, whole-body MRI, and/or 18F-NaF PET/CT can provide superior disease detection compared with conventional imaging and their results may alter patient management, although data are limited.
Also Check: Favorable Vs Unfavorable Intermediate-risk Prostate Cancer
Individuals Who Provided Content Development And/or Authorship Assistance:
Edward Schaeffer, MD, PhD, Panel Chair, has disclosed that he is a scientific advisor for AbbVie, Inc., and Janssen Scientific Affairs, LLC.
Sandy Srinivas, MD, Panel Vice Chair, has disclosed that she is a scientific advisor for Bayer HealthCare, and receives grant/research support from Bayer HealthCare, Endocyte, and Exelixis Inc.
Emmanuel S. Antonarakis, MD, Panel Member, has disclosed that he has received consulting fees from Amgen Inc., Astellas Pharma US, Inc., AstraZeneca Pharmaceuticals LP, Clovis Oncology, Dendreon Corporation, Eli Lilly and Company, GlaxoSmithKline, Janssen PharmaceuticaProducts, LP, Medivation, Inc., Merck & Co., Inc., and ESSA Pharma, Inc. received grant/research support from AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Celgene Corporation, Clovis Oncology, Dendreon Corporation, Genentech, Inc., Janssen PharmaceuticaProducts, LP, Johnson & Johnson, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Tokai, and sanofi-aventis U.S. and receives royalty income from Qiagen.
Xin Gao, MD, Panel Member, has disclosed that he has received honoraria from Exelixis Inc.
George Netto, MD, Panel Member, has disclosed that he has no relevant financial relationships.
Daniel E. Spratt, MD, Panel Member, has disclosed that he receives grant/research support from Janssen PharmaceuticaProducts, LP.
Dorothy A. Shead, MS, Senior Director, Patient Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Both Nccn And Aua Include Enzalutamide As An Option For Certain Patients With Mcspc Nmcrpc And Mcrpc Based On High
Enzalutamide is the first and only novel hormone therapy recommended by the National Comprehensive Cancer Network® as a Category 1 treatment option* for certain patients with mCSPC, nmCRPC, and mCRPC1
Metastatic CSPC
NCCN Clinical Practice Guidelines in Oncology recommend enzalutamide with androgen deprivation therapy as a Category 1 treatment option* for patients with mCSPC
Nonmetastatic CRPC
NCCN Guidelines® recommend enzalutamide with continued androgen deprivation therapy as a Category 1 treatment option* for patients with nmCRPC when PSADT is 10 months
Metastatic CRPC
NCCN Guidelines recommend enzalutamide with continued androgen deprivation therapy as a Category 1 first-line treatment option* for patients with mCRPC
*A Category 1 recommendation is based on high-level evidence and indicates uniform NCCN consensus that the intervention is appropriate.1
For patients with mCRPC and no prior docetaxel, and no prior novel hormone therapy.1
Enzalutamide is the first novel hormone therapy recommended with a Strong Recommendation* by the American Urological Association in mCSPC, nmCRPC, and mCRPC2
Metastatic CSPC
Clinical Oncology 3
Metastatic CRPC
Strong evidence supports the ASCO Guidelines recommendation of enzalutamide in addition to androgen deprivation therapy as a therapeutic option in mCRPC to improve survival and to provide a favorable benefit-risk profile
Please see NCCN , AUA , and ASCO guidelines for full recommendations
Select Safety Information
Indications
Read Also: How Many Stages Does Prostate Cancer Have
Bone Scan For Diagnosis Of Metastatic Disease
Current NCCN guidelines include scanning technology utilizing fluorine-18 sodium fluoride as the tracer for the subsequent positron-emission tomography scan as an option for men with prostate cancer who undergo a bone scan to search for metastatic disease. PET and hybrid imaging bone scans appear more sensitive than conventional 99-technetium bone scans.
Imaging In Advanced Prostate Cancer

Guidelines from the American Society of Clinical Oncology recommend imaging for all patients with advanced prostate cancer, using one or more of the following modalities, according to the clinical scenario :
- Conventional imaging Computed tomography , bone scan, prostate magnetic resonance imaging
- Next-generation imaging Positron emission tomography , PET/CT, PET/MRI, whole-body MRI)
Disease states and clinical scenarios should be taken into consideration when choosing an imaging modality, as the modality may guide treatment or change clinical treatment decisions.
You May Like: What Side Effects Does Having Your Prostate Removed
More Options For Metastatic Disease
Subsequent systemic therapy for metastatic CRPC has become more complicated. In 2018, the NCCN panel differentiated visceral metastases from skeletal metastases for the purpose of selecting therapy.
For patients without visceral metastases, first options include abiraterone with prednisone, docetaxel, enzalutamide , and radium-223 for symptomatic bone metastases referral to a clinical trial and secondary hormone therapy.
For patients with visceral metastases, the guideline recommends consideration of a biopsy, choosing subsequent therapy based on histologic evidence of small-cell carcinoma or adenocarcinoma, and then treating the patient according to the guideline.
Nccn Clinical Practice Guidelines In Oncology: 2020 Updates
In 1996, the National Comprehensive Cancer Network published its first set of Clinical Practice Guidelines in Oncology®, covering eight tumor types. Guidelines are now published for more than 60 tumor types and topics. During the NCCNs 25th Annual Conference, which was held virtually during the COVID-19 pandemic, key 2020 updates were presented for several tumor types, many of which are briefly presented here. We also want to emphasize the emerging recognition of cardio-oncology as an important aspect of cancer care and the changing landscape of acute myeloid leukemia. Weve summarized those presentations as well.
Metastatic Breast Cancer
The phase II Destiny-Breast01 trial results got everyone excited in San Antonio and led to the approval of fam-trastuzumab-deruxtecan by the U.S. Food and Drug Administration.
William J. Gradishar, MD
William J. Gradishar, MD
Updates in the systemic treatment of metastatic breast cancer were presented by William J. Gradishar, MD, the Betsy Bramsen Professor of Breast Oncology and Chief of Hematology/Oncology at the Robert H Lurie Comprehensive Cancer Center, Northwestern University, Chicago. The updates were few, but they should significantly improve the care of patients:
Metastatic Colon Cancer
The treatment paradigm for metastatic colorectal cancer has greatly changed in recent years. Its no longer just FOLFOX and FOLFIRI.
Dustin A. Deming, MD
Dustin A. Deming, MD
Rectal Cancer
Christopher G. Willett, MD
Pancreatic Cancer
You May Like: Can Lack Of Ejaculation Cause Prostatitis
Parp Inhibitors For Patients With Dna Repair Gene Mutations
Results of early studies suggest that germline and somatic mutations in homologous recombination repair genes may be predictive of the clinical benefit of PARP inhibitors.44â46 PARP inhibitors are oral agents that exert their activity through synthetic lethality.47 Currently, 2 PARP inhibitors, olaparib and rucaparib, are FDA-approved for use in prostate cancer.48,49 The panel discussed the FDA approvals and the data outlined below and voted to add olaparib and rucaparib to the guidelines in recent versions.
Cabazitaxel In Later Lines Of Therapy For Mcrpc
The panel also discussed results of the multicenter, randomized, open-label CARD study, which compared cabazitaxel with either abiraterone or enzalutamide in 255 patients with metastatic CRPC who had previously received docetaxel and either abiraterone or enzalutamide.21 Either order of the previously received therapies was allowed, and abiraterone or docetaxel could have been given in the castration-naïve setting. Disease progression on abiraterone or enzalutamide had to have occurred within 12 months for patients to be eligible. Cabazitaxel at 25 mg/m2 with concurrent steroid improved the primary endpoint of radiographic PFS and reduced the risk of death compared with abiraterone or enzalutamide in these patients . Cabazitaxel was also associated with an increased rate of pain response and delayed time to pain progression and skeletal-related events.22
Panel consensus was that results of CARD provide level 1 evidence supporting cabazitaxel over abiraterone or enzalutamide in patients who have already received docetaxel and either abiraterone or enzalutamide. Therefore, the panel included cabazitaxel as a category 1, preferred option for patients with prior docetaxel and prior novel hormone therapy in the metastatic CRPC setting .
Read Also: What Can You Do About Enlarged Prostate
European Society Of Medical Oncology
The 2015 ESMO guidelines recommend watchful waiting with delayed hormone therapy as an option for localized disease or as an alternative for men with localized or locally advanced disease who are unwilling or unsuited for radical therapy.
Other recommended treatment options include :
-
Active surveillance for men with low-risk disease
-
Radical prostatectomy or radiotherapy for men with low- or intermediate-risk disease
-
Primary androgen deprivation therapy alone is not recommended for treatment of non-metastatic disease
-
For patients with high-risk or locally advanced prostate cancer, external beam RT plus hormone treatment or RP plus pelvic lymphadenectomy
