Rising Psa After Radiotherapy And Negative Conventional Imaging
For men for whom salvage local or regional therapy is not planned or is inappropriate, there is little evidence that NGI will alter treatment or prognosis. The role of NGI in this scenario is unclear and it should not be offered, except in the context of an institutional review boardapproved clinical trial.
For men for whom salvage local or regional therapy is contemplated, evidence supports NGI for detection of local and/or distant sites of disease. Findings on NGI could guide management in this setting . PSMA imaging , 11C-choline or 18F-fluciclovine PET/CT or PET/MRI, whole-body MRI, and/or 18F-NaF PET/CT can provide superior disease detection compared with conventional imaging and their results may alter patient management, although data are limited.
Active Surveillance Again Preferred Choice For Most Men With Low
After protest by urologists and patient advocates, the prostate cancer panel of the National Comprehensive Cancer Network reversed itself on Tuesday, with new guideline language restoring active surveillance as a preferred approach for “most” men with low-risk prostate cancer.
In September, the NCCN panel had published guidelines that eliminated the word “preferred” for AS in the low-risk group, putting it on par with radical prostatectomy and radiotherapy. All three approaches offer similar life expectancy, but AS eliminates quality-of-life issues experienced with the other approaches, such as incontinence and impotence.
“Writing NCCN guidelines is an iterative process,” panel chair Edward Schaeffer, MD, PhD, of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago, said in an interview. “I am glad to have shepherded these new guidelines through. This has been an absolutely positive experience.”
The one-word change in September unleashed a twitter-storm among urologists who felt the action was unwarranted after 25 years of research establishing AS as a preferred treatment.
Matthew Cooperberg, MD, of the University of California San Francisco, who led the twitter-storm, called the September change a “step in the wrong direction,” but praised the latest revision by the NCCN, the leading group for guidelines for prostate cancer.
New Perspective On Low
The paradigms of prostate cancer detection are changing so that detection of low-risk disease is not followed, and surveillance rather than treatment is offered to those with lower-risk disease, Dr Carroll said. This sentiment was echoed by the chair of the NCCN Prostate Cancer Guideline Committee, James L. Mohler, MD, Associate Director, Translational Research, Roswell Park Comprehensive Cancer Center, Buffalo, NY, who discussed management approaches in prostate cancer.
An alternative to performing biopsies in patients with elevated prostate-specific antigen levels is the use of serum- or urine-based biomarkers that increase the specificity of screening.
What theyre doing is determining which men with an elevated PSA are harboring clinically significant disease, defined by an elevated Gleason score, said Dr Carroll. These tests miss few high-risk cancers, but decrease the biopsy rate by 30% to 40%, he added.
The other big marker right now is multiparametric MRI , Dr Carroll told attendees. Using multiparametric MRI or biomarkers misses only approximately 1% to 2% of high-risk tumors, and even fewer if both tests are used, while avoiding unnecessary biopsies and detecting fewer lower-risk cancers.
In my opinion, very few men with low-risk disease should ever be treated, Dr Carroll posited. Several studies have shown that there is no harm in delaying treatment by up to 2 years, he noted.
Also Check: Can Your Prostate Cause Testicle Pain
Nccn Removed Preferred Status For Active Surveillance In Low
The National Comprehensive Cancer Network is under fire for removing active surveillance’s “preferred” status in its low-risk prostate cancer guidelines.
AS has been listed as preferred since 2019, and the change puts it on par with radical prostatectomy and radiation therapy in this patient population.
Edward “Ted” Schaeffer, MD, PhD, chairman of the NCCN prostate cancer guidelines panel, defended the change as a “minor adjustment” that will serve patients by encouraging more discussion of options for low-risk patients.
“My personal feeling is that there is more nuance as the risk groups increase,” said Schaeffer, of Northwestern University’s Feinberg School of Medicine in Chicago. “Each individual case should be more strongly reviewed and discussed with patients, and patients should be involved in the shared decision-making process. I don’t feel there is any change in the guidelines. Active surveillance is still listed first, and it’s still listed as an option.”
He stressed that the guidelines, dated September 10, still hold that AS is “preferred” for very-low risk prostate cancer.
Matthew Cooperberg, MD, a urology professor at the University of California San Francisco , sees the change as “a step backward” in the quarter century campaign to reduce overdiagnosis and overtreatment of low-risk prostate cancer.
Patient groups were divided about the potential impact on their constituents.
Sequencing Of Systemic Therapy For Crpc
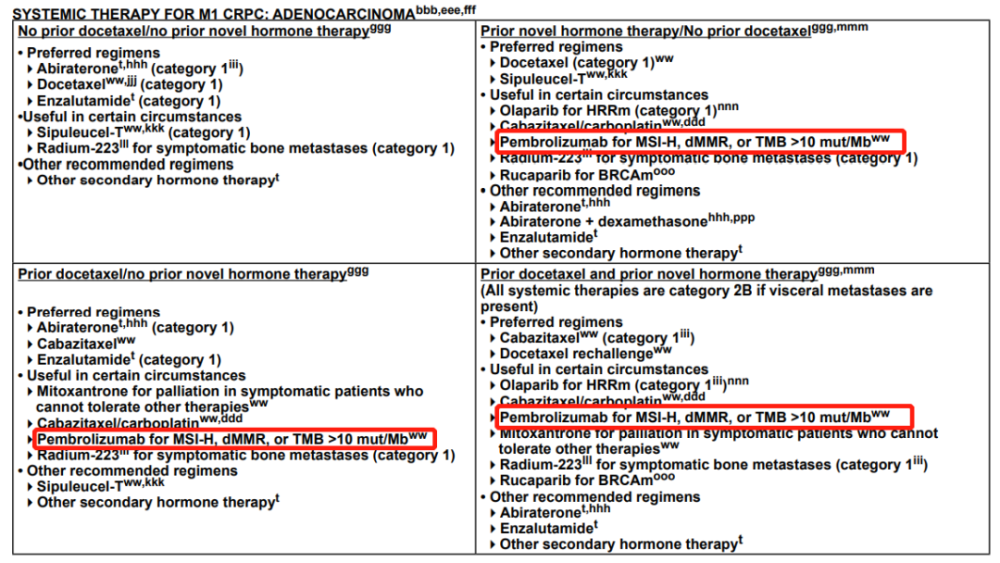
Systemic therapies for patients with CRPC include various secondary hormone therapies, chemotherapies, immunotherapies, radiopharmaceuticals, and/or targeted therapies. Specific options, as delineated in the guidelines for patients with and without distant metastases, are based on a large body of data. However, a limited amount of data informs the optimal sequence for delivery of these agents. Choice of treatments in various lines of therapy is based on patient preferences, prior treatment exposures, the presence or absence of visceral disease, patient symptoms, and potential side effects.
In all cases, patients experiencing disease progression on a given therapy should not repeat that therapy, with the exception of docetaxel, which can be given as a rechallenge in the metastatic CRPC setting after progression on a novel hormone therapy if given in the castration-naive setting without definitive evidence of progression.
You May Like: Why Is My Prostate Enlarged
Parp Inhibitors For Patients With Dna Repair Gene Mutations
Results of early studies suggest that germline and somatic mutations in homologous recombination repair genes may be predictive of the clinical benefit of PARP inhibitors.44â46 PARP inhibitors are oral agents that exert their activity through synthetic lethality.47 Currently, 2 PARP inhibitors, olaparib and rucaparib, are FDA-approved for use in prostate cancer.48,49 The panel discussed the FDA approvals and the data outlined below and voted to add olaparib and rucaparib to the guidelines in recent versions.
Somatic Tumor Testing Based On Risk Groups
NCCN recommendations for testing of prostate cancer tumors are as follows:
-
Tumor testing for homologous recombination gene mutations and for microsatellite instability or mismatch repair deficiency can be considered in patients with regional prostate cancer.
-
Tumor testing for somatic HRRm is recommended in patients with metastatic prostate cancer.
-
Multigene molecular testing can be considered for patients with low- and favorable-intermediate risk prostate cancer and life expectancy 10 years.
-
The Decipher molecular assay can be considered as part of counseling for risk stratification in patients with PSA resistance/recurrence after radical prostatectomy.
-
If mutations in BRCA2, BRCA1, ATM, CHEK2, or PALB2 are found, the patient should be referred for genetic counseling to assess for the possibility of hereditary breast and ovarian cancer syndrome.
-
If MSI testing is performed, testing using an NGS assay validated for prostate cancer is preferred. If high MSI or dMMR is found, the patient should be referred for genetic counseling to assess for the possibility of Lynch syndrome. MSI-H or dMMR indicate eligibility for pembrolizumab in second and subsequent lines of treatment of castration-resistant prostate cancer.
Don’t Miss: How To Relieve Pain From Prostatitis
American Society Of Clinical Oncology/cancer Care Ontario Recommendations
ASCO and CCO released a joint clinical practice guideline for treatment of men with metastatic CRPC in 2014. The guideline recommendations include the following :
-
Pharmacologic androgen deprivation therapy should be continued indefinitely
-
Offer patients one of three treatment optionsabiraterone/prednisone, enzalutamide, or radium-223 in addition to hormone deprivation
-
When considering chemotherapy, docetaxel/prednisone should be an option but side effects must be discussed
-
Offer cabazitaxel to men whose disease worsens even if docetaxel has been tried, but again, discuss side effects
-
Offer sipuleucel-T to men with no symptoms or minimal symptoms of cancer
-
Offer mitoxantrone, but include a discussion of the drug’s limited clinical benefit and side effect risk
-
Offer ketoconazole or the anti-androgen therapies bicalutamide, flutamide or nilutamide but discuss the limited clinical benefit for these three medications
-
Do not offer the drugs bevacizumab , estramustine, or sunitinib
-
Begin discussion of palliative care early on while discussing treatment options
Cancer Care Ontario/american Society Of Clinical Oncology
In 2016, the American Society of Clinical Oncology endorsed Cancer Care Ontarios guideline on active surveillance for the management of localized prostate cancer. The recommendations include the following:
- Active surveillance is the recommended disease management strategy for most patients with lowrisk localized prostate cancer.
- Because of heterogeneity within this population, factors such as younger age, high-volume Gleason 6 cancer, patient preference, and/or African American ethnicity should be taken into account in the decision to use active surveillance.
- Young patients with high-volume Gleason 6 cancer should be closely scrutinized for the presence of highergrade cancer definitive therapy may be warranted for select patients.
- For patients with limited life expectancy and lowrisk cancer, watchful waiting may be more appropriate than active surveillance.
- Active treatment is recommended for most patients with intermediaterisk localized prostate cancer, but active surveillance may be offered to select patients with lowvolume, intermediaterisk localized prostate cancer.
The guidelines recommend that the active surveillance protocol include the following tests:
Read Also: Can A Swollen Prostate Affect Bowel Movements
American Urological Association Recommendations
American Urological Association guidelines for the management of CRPC describe six index-patient scenarios for which recommendations could be formulated.
Index patient no. 1: Asymptomatic non-metastatic CRPC
Recommendations are as follows:
-
Observation with continued ADT
-
First-generation antiandrogens or first-generation androgen-synthesis inhibitors to patients unwilling to accept observation.
-
Systemic chemotherapy or immunotherapy should not be offered to patients with non-metastatic CRPC outside the context of a clinical trial
Index patient no. 2: Asymptomatic or minimally-symptomatic, metastatic CRPC with good performance status and without prior docetaxel chemotherapy
Recommendations are as follows:
-
Abiraterone plus prednisone, enzalutamide, docetaxel, or sipuleucel-T
-
First-generation antiandrogen therapy or ketoconazole plus steroid or observation to patients who do not want or cannot have one of the standard therapies
Index patient no. 3: Symptomatic, metastatic CRPC with good performance status and no prior docetaxel chemotherapy
Recommendations are as follows:
-
Docetaxel
-
Abiraterone plus prednisone, enzalutamide, or docetaxel
-
Ketoconazole plus steroid, mitoxantrone, or radionuclide therapy for patients who do not want or cannot have one of the standard therapies
-
Radium-223 to patients with symptoms from bony metastases and without known visceral disease
-
Treatment with either estramustine or sipuleucel-T should not be offered
Recommendations are as follows:
Individuals Who Provided Content Development And/or Authorship Assistance:
Edward Schaeffer, MD, PhD, Panel Chair, has disclosed that he is a scientific advisor for AbbVie, Inc., and Janssen Scientific Affairs, LLC.
Sandy Srinivas, MD, Panel Vice Chair, has disclosed that she is a scientific advisor for Bayer HealthCare, and receives grant/research support from Bayer HealthCare, Endocyte, and Exelixis Inc.
Emmanuel S. Antonarakis, MD, Panel Member, has disclosed that he has received consulting fees from Amgen Inc., Astellas Pharma US, Inc., AstraZeneca Pharmaceuticals LP, Clovis Oncology, Dendreon Corporation, Eli Lilly and Company, GlaxoSmithKline, Janssen PharmaceuticaProducts, LP, Medivation, Inc., Merck & Co., Inc., and ESSA Pharma, Inc. received grant/research support from AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Celgene Corporation, Clovis Oncology, Dendreon Corporation, Genentech, Inc., Janssen PharmaceuticaProducts, LP, Johnson & Johnson, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Tokai, and sanofi-aventis U.S. and receives royalty income from Qiagen.
Xin Gao, MD, Panel Member, has disclosed that he has received honoraria from Exelixis Inc.
George Netto, MD, Panel Member, has disclosed that he has no relevant financial relationships.
Daniel E. Spratt, MD, Panel Member, has disclosed that he receives grant/research support from Janssen PharmaceuticaProducts, LP.
Dorothy A. Shead, MS, Senior Director, Patient Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Recommended Reading: How Deep Is Prostate Gland
Updates Of Changes In The Early Detection Of Prostate Cancer Nccn Guidelines 2021 Summary
The Virtual Global Summit on Precision Diagnosis and Treatment of Prostate Cancer brings together key international opinion leaders of every clinical subspecialty involved in patient care. This event is an integral part of the AdMeTech Foundations Annual Summit, which was established in 2016 and became seminal in shaping the state of the art and future vision for precision care. The goal of this event is three-fold: 1) Educating the key stakeholders 2) Supporting a sustained cross-disciplinary dialogue and consensus on the best emerging clinical practices and research priorities and 3) Expediting clinical adoption of promising novel diagnostics and therapeutics. For more educational activities from this virtual event, visit our collection page.
Nccn Guidelines Add Psma
The NCCN has added Ga 68 and F 18based PSMA-PET imaging modalities to its clinical practice guidelines for prostate cancer.1
The updated guidelines will encourage clinicians to use PSMA-PET as a primary imaging modality in patients and will deliver the benefit of a more streamlined approach. We look forward to having access to this functional form of imaging as new products come into the market, Oliver Sartor, MD, Medical Director at Tulane Cancer Center, stated in a news release from Telix pharmaceuticals, a manufacturer of PSMA-PET imaging products.
According to the release, The NCCN panel has recognized the increased sensitivity and specificity of PSMA-PET tracers, compared to conventional imaging for detecting micrometastatic disease, at both initial staging and biochemical recurrence. The updated guidelines state that the NCCN Panel does not feel that conventional imaging is a necessary prerequisite to PSMA-PET and that PSMA-PET/CT or PSMA-PET/MRI can serve as equally effective, if not more effective front-line imaging tools for these patients.1
Recommended Reading: Can Prostate Cancer Symptoms Come And Go
Intermittent Or Continuous Adt
In patients with castration-naïve prostate cancer, complete androgen blockade does not work, Dr Mohler said. Intermittent ADT is as good as continuous ADT in terms of overall survival , with a significant quality-of-life benefit. Intermittent ADT can be personalized based on the PSA response, Dr Mohler said. A PSA level < 0.2 ng/mL signals an outstanding response to ADT and a patient who will do well without ADT for a prolonged period.
For younger and healthier men with metastatic, castration-naïve prostate cancer, survival is improved with the addition of docetaxel to ADT. Abiraterone is a less toxic alternative to chemotherapy, with a similar effect on survival.
This has produced a change in the NCCN guideline, where we make more options available for castration-naïve disease, Dr Mohler said.
If the patient is asymptomatic, I always use intermittent ADT. I discuss a possible decrease in OS, but the trade-off is improved quality of life during off cycles, he added. Continuous ADT can be considered for men with symptomatic, castration-naïve disease, unless the PSA response is outstanding, in which case intermittent ADT is preferred.
Newly Diagnosed Clinically High
When conventional imaging is negative in patients with a high risk of metastatic disease, NGI may add clinical benefit, although prospective data are limited.
When conventional imaging is suspicious or equivocal, NGI may be offered to patients for clarification of equivocal findings or detection of additional sites of disease, which could potentially alter management, although prospective data are limited.
Read Also: What Causes An Enlarged Prostate In A Young Man
National Comprehensive Cancer Network Recommendations
The NCCN guidelines for prostate cancer include treatment recommendations for CRPC based on the presence or absence of visceral metastases. For the most part, these recommendations are based on high-level evidence and are supported by uniform NCCN consensus .
CRPC without distant metastasis
-
Enrollment in clinical trial is preferred
-
Observation is acceptable
-
Secondary hormone therapy can be considered for patients with prostate-specific antigen doubling < 10 months anti-androgen therapy is acceptable for patients who previously received medical or surgical castration, ketoconazole, corticosteroids, diethylstilbestrol or other estrogens
CRPC with bone metastases
Measures to promote bone health include the following:
-
Zoledronic acid or denosumab
-
Avoidance of invasive dental surgery during treatment
-
Calcium and vitamin D supplements to prevent hypocalcemia during treatment
Radium-233 can be used to treat symptomatic bone metastases without visceral metastases.
Metastatic CRPC with no visceral metastases
-
Sipuleucel-T for asymptomatic or minimally symptomatic patients
-
Abiraterone plus prednisone or enzalutamide for asymptomatic patients
-
Docetaxel with prednisone for symptomatic patients may also be considered in a symptomatic patients with signs of rapid progression
-
Radium-233 for symptomatic patients
-
Secondary hormone therapy or enrollment in clinical trial may be considered
Metastatic CRPC with visceral metastases
Metastatic CRPC with pathogenic mutations
Imaging In Advanced Prostate Cancer
Guidelines from the American Society of Clinical Oncology recommend imaging for all patients with advanced prostate cancer, using one or more of the following modalities, according to the clinical scenario :
- Conventional imaging Computed tomography , bone scan, prostate magnetic resonance imaging
- Next-generation imaging Positron emission tomography , PET/CT, PET/MRI, whole-body MRI)
Disease states and clinical scenarios should be taken into consideration when choosing an imaging modality, as the modality may guide treatment or change clinical treatment decisions.
You May Like: How Often Should You Test For Prostate Cancer
Nccn Guidelines: Updating Initial Risk Stratification And Staging Workup For Clinically Localized Evaluation Of Prostate Cancer
The latest update to the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Prostate Cancer, Version 2.2022, was issued on November 30, 2021. It followed only 2 months after the September 30 release of Version 1.1022,2 which caused controversy by removing preferred option to describe active surveillance in patients with low-risk prostate cancer. There are challenges in keeping this guideline up to date, because there are so many amazing changes taking place in prostate cancer, according to NCCN Prostate Cancer Panel chaired by Edward Schaeffer, MD, PhD, Chair of Urology at Northwestern Memorial Hospital and Professor of Urology at Northwestern University, Chicago.3
In the section of the guideline that addresses initial risk stratification and staging workup for clinically localized disease , the biggest changes in Version 2.2022 from Version 1.2022 are in the utility of imaging, such as multiparametric magnetic resonance imaging , and the use of tumor multigene molecular testing in the very low-, low-, and intermediate-risk groups.
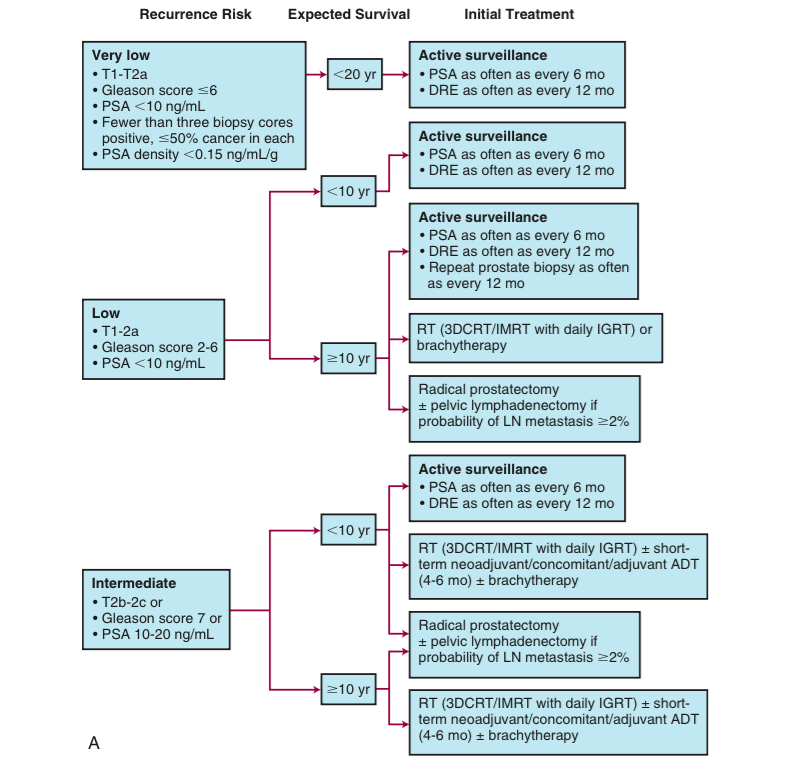
Very Low Risk Group
Low Risk Group
Intermediate Risk Group
Evidence for mpMRI and tumor multigene molecular testing
The NCCN recommendation to use tumor molecular testing is based on the goal of achieving personalized or precision medicine. Molecular testing of a tumor offers the potential to evaluate the biologic behavior of a cancer, which would aid in clinical decision making, the guideline says.
