Effects Of Radiation Therapy On Immunogenic Cell Death And Immune Anti
Radiation therapy increases DNA damage and leads to activation of IFN I response with pro-inflammatory effects and activation of T cells . Radiotherapy also increases antigen presentation . Indeed, radiotherapy induces immunogenic cell death, which allows the formation of TAA . These TAAs are captured by APCs such as DCs and presented to T cells via the major histocompatibility complex -I complex, with co-stimulatory signals such as CD80 . One of these TAA is the oncofetal tumor antigen 5T4, which is increased by irradiation. This leads to an enhancement of phagocytosis of irradiated tumor cells by DC and thus to an increase in cross-presentation of the 5T4 antigen to CD8+ T cells . The number of tumor-specific T cells is increased by radiotherapy in patients . In the ORIOLE study, significant clonotypic expansion after SABR was detected by sequencing the T cell receptor . A study by Berstein et al. investigated the effects of single dose EBRT on the modulation of costimulatory and co-inhibitory T cell molecules in PCa cell lines . The authors observed that irradiation increased the expression of OX40L , 4-1BBL and ICOSL , some of the T cell costimulatory molecules. Furthermore, 72h after irradiation, a decrease in PD-L1 and CTLA-4 expression were observed, as well as an increase in CD8+ T cell activity after their interaction with tumor cells. Thus, irradiation leads to an increase in the expression of co-stimulatory molecules and a decrease of co-inhibitory molecules.
Studying Exceptional Prostate Cancer Patient Responders
Study of exceptional responses at the molecular level with the volunteerism of prostate cancer patients has been overwhelmingly hypothesis generating. Graff and colleagues reported studies on an mCRPC patient who is in his sixth year of a complete clinical prostate cancer response to treatment with only three cycles of ipilimumab after experiencing autoimmune hepatitis. The patient mounted IgG responses to 11 candidate tumor-associated antigens, in particular to 3-hydroxyisobutyryl-CoA hydrolase .
Possible Side Effects Of Vaccine Treatment
Common side effects from the vaccine can include fever, chills, fatigue, back and joint pain, nausea, and headache. These most often start during the cell infusions and last no more than a couple of days. A few men may have more severe symptoms, including problems breathing and high blood pressure, which usually get better after treatment.
Recommended Reading: Can Herniated Disc Cause Prostate Problems
Combination Immunotherapy Benefits Subset Of Patients With Advancedprostate Cancer
Phase II trial provides rationale for pursuing further study of dualcheckpoint blockade
MD Anderson News ReleaseSeptember 10, 2020
Results from a Phase II trial led by researchers at The University of Texas MD Anderson Cancer Center suggest that a combination of ipilimumab plus nivolumab can generate durable responses in a subset of patients with metastatic castration-resistant prostate cancer , an immune-cold cancer that does not typically respond well to immunotherapy.
In a cohort of patients without previous chemotherapy treatment, the overall response rate was 25% and median overall survival was 19 months. In a post-chemotherapy cohort, the ORR was 10% and media OS was 15.2 months. Four patients achieved a complete response.
The results of the CheckMate 650 trial, published today in Cancer Cell, are the first report of combination immune checkpoint inhibitors in mCRPC. Early results from this study were presented at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium. Based on the findings, alternate dosing regimens are now being evaluated in an expanded clinical trial to reduce treatment-related toxicities.
Designing a combination strategy
This would explain why previous clinical trials evaluating single-agent checkpoint inhibitors have not been effective in treating patients with mCRPC, said Sharma, who co-directs MD Andersons immunotherapy platform, part of the institutions Moon Shots Program®.
A Brief Summary Of Study Participation
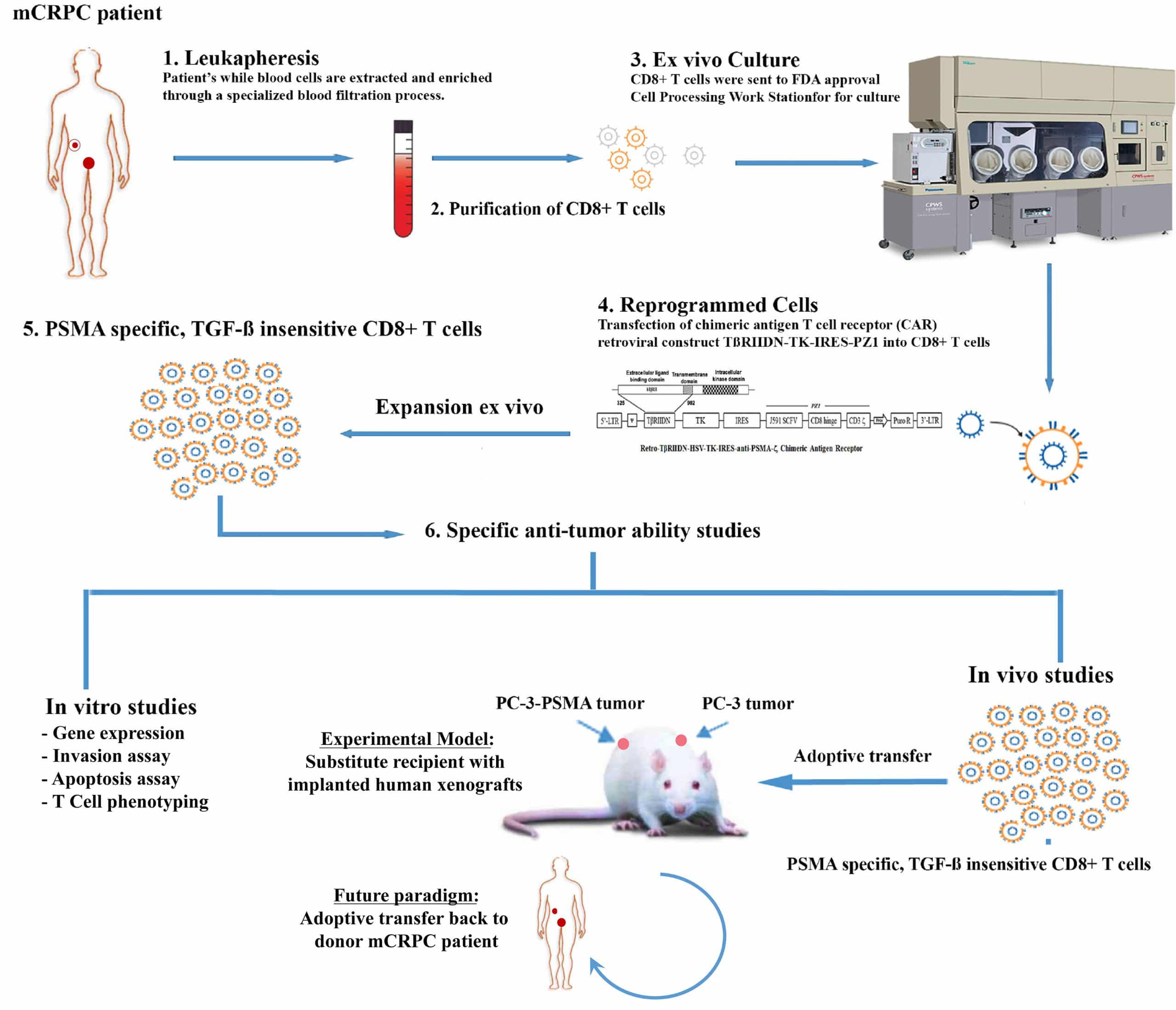
Participation in this study will include nine study visits over a period of approximately five months, with a Follow-Up Visit every six months until the end of the study.
Study participation is divided into three types of study visits:
- Screening Visit
More information will be provided to eligible participants regarding the study visit regimens.
Don’t Miss: How To Massage His Prostate
Immunotherapy In Prostate Cancer
Immune checkpoint inhibitors are antibodies designed to activate an effective immune response by targeting negative regulators of T cells such as PD-L1, PD-1 or CTLA-4 . The use of a CTLA-4-targeted monotherapy, known as ipilimumab, was tested in PCa in an unselected population, but did not result in significant benefit . This could be explained by increased expression of PD-1/PD-L1 as a compensatory mechanism that maintains inhibition of the T cell response . Alternatively, an anti-PD-1, pembrolizumab, has been commercialized for mCRPC with mismatch repair deficiency and/or microsatellite instability , although the relevance of this ICI is still debated. Indeed, the Keynote 19 trial demonstrated that pembrolizumab monotherapy induced antitumor activity in only a small number of mCRPC patients, with an objective response rate up to 5% . However, in a phase 2 trial , double-blockade immunotherapy with nivolumab and ipilimumab showed an ORR of 26% in asymptomatic or minimally symptomatic patients with mCRPC . Therefore, determining the subpopulations that might benefit from ICIs immunotherapy appears essential.
Introduction: Prostate Cancer Responds To And Warrants Immunotherapy Research
Now this is not the end. It is not even the beginning of the end. But it is, perhaps, the end of the beginning.
Sir Winston Churchill
Prostate cancer research is an exciting, compelling, and fundable area of biomedical science . Although metastatic, hormone therapyrefractory prostate cancer remains the second most common cause of cancer-related deaths in men after lung cancer in the United States, the years of life lost to prostate cancer have dropped by 40% in the past 20 years . In total, six new agents each with its own new and different mechanism of actionhave been approved by the FDA for treatment of metastatic prostate cancer . More new FDA-approved drugs have been used for treatment of mPCA in the past 3 years than in the past four decades. For patients beyond early detection and cure with surgery or radiotherapy, survival times for men with mPCA have been more than doubled, with a new armamentarium of systemic treatments .
Read Also: Can Prostate Issues Cause Erectile Dysfunction
Prostate Tumor Cells Express Immune Checkpoint Ligands
To escape the anti-tumor immune response, tumor cells increase their expression of immune checkpoint ligands, such as PD-L1. This molecule binds to its receptor, programmed cell death 1 , which is expressed by T cells, leading to their anergy. Patients with expression of at least 1% of PD-L1 on tumor cells are associated with shorter metastasis-free survival than those with PD-L1 negative tumors . Furthermore, these patients have a fourfold higher risk of developing distant metastases. Another negative regulator of T cells is the cytotoxic T lymphocyte antigen 4 , which is also upregulated in PCa .
Where We Have Come From And Where We Are Now
Science must begin with myths and with the criticisms of myths.
Karl Popper
The first two decades of molecular immunotherapy research in prostate cancer were focused fundamentally on dispelling myths. The central 20th-century myth was that prostate cancer in the clinic could never respond to immunotherapy. The Bethesda nihilism myth was predicated on these axioms: Unlike melanoma or renal carcinoma, immune-mediated spontaneous regressions and abscopal effects in mCRPC never occurred in patients the prostate gland was intrinsically nonimmunogenic: peripheral tolerance could never be broken older men with mCRPC had preexisting T-cell anergy due to aging that prevented antineoplastic T-cell immune responses all prostate cancers were MHC class Ilow/null and no antitumor activity was seen using recombinant cytokines as single agents, e.g., INF or IL2, in small clinical trials. Although evidence existed that men with untreated prostate cancer had active delayed-type hypersensitivity responses to autologous tumor antigens derived from lysates of their own cancers, the data were in small numbers from a single institution’s databuried in the clinical urology literature of the 1970s .
Don’t Miss: Nccn Guidelines Prostate Cancer 2021
What Are The Signs That Immunotherapy Is Working
Immunotherapy is a type of treatment that has been shown to successfully treat a wide variety of cancers. However, it is not without side effects. Although most of them will go away once the treatment is over, some may persist for months or even years. It is important to report any unpleasant side effects to your healthcare provider.
The first sign that immunotherapy is working is a decrease in the size of the tumor. Sometimes, the tumor will appear larger on scans before shrinking. This is a side effect called pseudoprogression. This does not necessarily mean that your treatment is not working, but it is a common side effect. In this case, your care team may suggest that you wait a couple of more treatment cycles before having a confirmatory scan to see if immunotherapy is improving your condition.
A second sign is an increase in the prostate-specific antigen. This sign occurs when the cancer has spread to distant organs. This can occur even with no other cancer symptoms.
Vaccines With Immune Checkpoint Inhibitors
Immune check point inhibitors have significantly failed in improving clinical outcome in prostate cancer patients. There was no improvement in OS with ipilimumab and nivolumab, but the progression free interval improved. It depicts that, there was response, but not up to that extent to become statistically significant. The other likely reason for failure of immune checkpoint inhibitors is not enough inflammation and T cell infiltration in microenvironment of the tumor to cause immune activation and destruction of cancer cells. Vaccine on the other hand didnt improve PFS but OS improved and with Sipuleucel-T, there is increase in T cell infiltration and inflammation in microenvironment of tumor. Therefore, there should be synergistic effect in combination of vaccines and immune checkpoint inhibitors.
A clinical trial of combination of ipilimumab and therapeutic cancer vaccine Prostvac has shown preliminary evidence of improvement in clinical and immunologic outcome. The median OS was 34.4 months which as compared to Prostvac alone in contemporary study was 26.3 months . There was reduction in PSA in 54% of patients and PSA decline more than 50% was seen in 25% of patients. This study suggests potential synergy between vaccines and Immune check point inhibitors. Further studies are going on combination therapy of vaccines and immune checkpoint inhibitors which have been shown in Tables 1-5.
Read Also: Prostate Cancer Treatment Guidelines 2020 Nccn
Checkpoint Inhibitors: Why Not Prostate Cancer
The excitement over immunotherapy in solid tumors has occurred as a result of the significant and durable responses obtained in several malignancies using checkpoint inhibitors. The first, a monoclonal antibody directed against the checkpoint molecule, CTLA-4 was approved for melanoma in the setting of improved survival and antitumor effects. CTLA-4 is a protein receptor that resides within the T cells and downregulates the immune system . Upon T-cell engagement with dendritic or antigen presenting cells , the cells that present cancer or foreign antigens to the T cell, the T cell must have certain costimulatory molecules that tell it to either proliferate or abort its interaction with the APC. Activation of resting or quiescent T-cell requires two complementary signals. Engagement of the T-cell receptor must be accompanied by a second signal that results from the binding of receptors on the T cell with either soluble factors, such as IL-2, or cell-surface molecules on the antigen-presenting cell. CD28 and CTLA-4 are receptors on T cells that play critical roles in the initial activation and subsequent control of cellular immunity. CD28 is expressed constitutively on T cells it provides a co-stimulatory signal upon binding to target ligands on antigen-presenting cells. Conversely, CTLA-4 is transiently expressed following T-cell activation. The signal delivered through CTLA-4 down regulates T-cell function and inhibits excessive expansion of activated T cells.
Parp Inhibition In Prostate Cancer
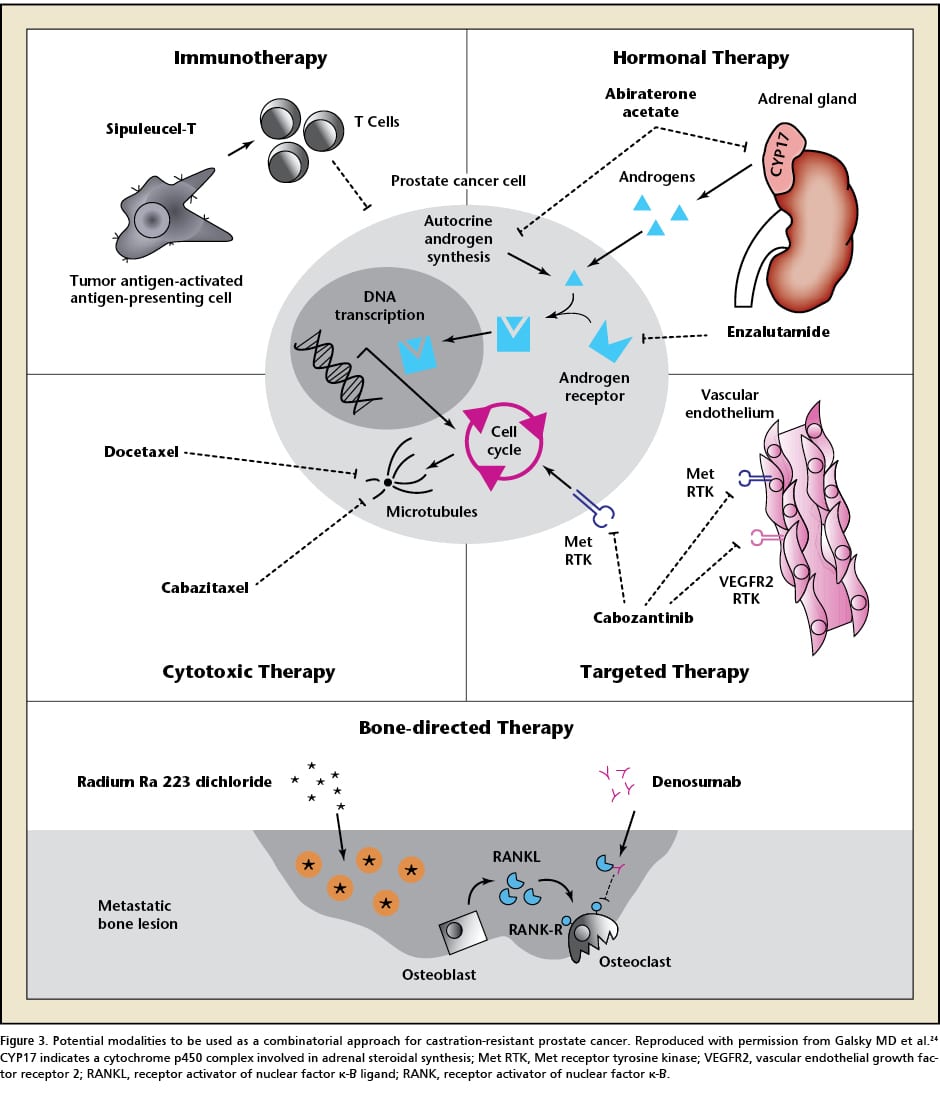
PARP is an enzyme which initiate single stranded DNA repair. In patient with underlying DNA repair problem, if we inhibit PARP there will be no DNA repair and cell will die. This is the basic principle of action of PARP inhibitors. One study showed that genomic testing from bone and soft tissue metastasis of CRPC has DNA repair alterations. There are aberrations in androgen receptor , TP53, PTEN, BRCA1/2 and ATM genes. It also showed that 89% of these are clinically actionable DNA mutation and frequency of these aberrations increase with progression of disease . Olaparib is an oral PARP inhibition, which is approved for BRCA1/2 mutant ovarian cancer . As prostate cancer patients also have this mutation, active PARP inhibition can be a promising treatment. In a phase 2 study where mCRPC patients not responding to standard therapy are treated with Olaparib and mutational status was assessed by genomic sequencing. It showed that 33% patients have high response to Olaparib therapy and it was associated with defect in DNA repair gene. BRCA and ATM mutations were found in 10% and 12% of patients respectively, out of 49 patients. All patients with BRCA mutations and 80% of ATM mutations had clinical response to Olaparib. It was a small study but it prompted a new therapy for CRPC .
Table 5
Recommended Reading: What Happens When Your Prostate Is Removed
Does Immunotherapy Stop Cancer Spreading
There is a new type of treatment for prostate cancer that uses the patients own immune system to fight the disease. It is FDA-approved to treat some forms of this disease. The treatment aims to kill tumor cells with fewer doses over a shorter time period, causing less damage to normal tissue. While chemotherapy is an effective option, many patients find it more comfortable to use a natural approach that relies on the bodys own immune system.
While immunotherapy has made significant advances in the treatment of melanoma and lung cancer, it is still a relatively new treatment for prostate cancer. While there are a few approved therapies, most have yet to show significant clinical results. In addition to vaccine immunotherapy, other therapies are being researched. For example, there is active research on new immunotherapies targeting PARP inhibition and VISTA, as well as the combination of different types of immune cells. These therapies may one day be approved as a treatment for advanced cancer.
There are a few challenges associated with immunotherapy, but it has been shown to improve survival rates in patients with advanced prostate cancer. The most common treatment is radiation, but immunotherapy has the added advantage of using the patients own immune system to fight the disease.
Immunoevasion And New Targets Of Treatment Opportunity
Prostate cancer evades host immune responses resulting in approximately 29,000 deaths per year in the United States. While peritumoral CD8+ cells are often seen, robust tumor-infiltrating lymphocytes are rarely observed in primary prostate cancer tumors or in AR+, PSA+, HIF1+ mCRPC biopsies of metastases . When heavily pretreated mCRPC patients have preterminal tumor burdens on bone scans , a significant percentage of these patients have TCR loss in their peripheral blood T cells . In contrast, TCR is not downregulated in tumors with a high Gleason grade at diagnosis . CD4+ prostate-infiltrating lymphocytes in patients show a dearth of Th2-IL4secreting cells and appear more skewed toward a FoxP3+ T-regulatory cell and Th17 phenotypes . However, between diagnosis and death from prostate cancer, in situ CD8+ T cells in the human prostate cancer microenvironment are metabolically intact, but apparently they are refractory to activation even for allo-responses . Although CD8+ cells can infiltrate and traffic to prostate cancer tumors, there are TCR distal blocks for anti-neoplastic CD8+ killing. Dissecting these blocks and then trying to drug each block individually and in cotargeting strategies are at the vanguard of the prostate cancer immune-oncology research agenda.
Recommended Reading: What Is The Leading Cause Of Prostate Cancer
Interaction Between Modern Radiotherapy And Immunotherapy For Metastatic Prostate Cancer
- 1Institut de Cancérologie de lOuest, Nantes, France
- 2Université de Nantes, CNRS, Inserm, CRCINA, Nantes, France
Prostate cancer is the most frequently diagnosed cancer in men and a leading cause of cancer-related death. In recent decades, the development of immunotherapies has resulted in great promise to cure metastatic disease. However, prostate cancer has failed to show any significant response, presumably due to its immunosuppressive microenvironment. There is therefore growing interest in combining immunotherapy with other therapies able to relieve the immunosuppressive microenvironment. Radiation therapy remains the mainstay treatment for prostate cancer patients, is known to exhibit immunomodulatory effects, depending on the dose, and is a potent inducer of immunogenic tumor cell death. Optimal doses of radiotherapy are thus expected to unleash the full potential of immunotherapy, improving primary target destruction with further hope of inducing immune-cell-mediated elimination of metastases at distance from the irradiated site. In this review, we summarize the current knowledge on both the tumor immune microenvironment in prostate cancer and the effects of radiotherapy on it, as well as on the use of immunotherapy. In addition, we discuss the utility to combine immunotherapy and radiotherapy to treat oligometastatic metastatic prostate cancer.
Treating Castrate Resistant Metastatic Disease
The standards of care continue to be initiating first and second line antiandrogens i.e., the addition of antiandrogens or AR-directed therapies in the setting of patients who have been on single agent agonist or antagonists or have been on prior antiandrogens. For the latter, a trial of antiandrogen withdrawal is reasonable. It should be noted that following a standard treatment algorithm for all patients may not be reasonable as these patients may have a more aggressive biology and need other means of evaluating the potential of the behavior, i.e., gene profiling of tumor or assessing circulating tumor cells.
When to initiate docetaxel in this setting remains the physician’s choice. The rationale for using docetaxel first line after standard hormonal therapies in lieu of its immediate use posthormonal therapy may be based on several factors. Patients whose prostate-specific antigen is rapidly rising and unresponsive to first-line hormonal therapy are more symptomatic, i.e., failure to thrive, poor oral intake, decreased performance status, or having multiple sites of pain whereby significant radiation would be needed are appropriate candidates to start docetaxel. Not only was a survival benefit observed but it also improved the quality of life. Determining the biology through the disease’s natural history is important in determining how to proceed with any given therapy.
Don’t Miss: What Age Does Prostate Problems Occur
