Cbd Oil And Cancer: 9 Things To Know
CBD oil is everywhere these days. Once available only at novelty or vitamin shops, its now also at your local grocery store, pharmacy or even yoga studio.
It comes in many forms: oils that are dropped under the tongue, roll-ons that are applied to the skin and even solutions for vaping. Some producers extract CBD oil and add it into foods to create edible products.
But what is CBD oil exactly, and how does it affect cancer patients? Can it really treat or even cure cancer or relieve its symptoms? To separate fact from fiction, we spoke with our Kimberson Tanco, M.D. Heres what he wants cancer patients to know.
What is CBD oil, and how does it differ from marijuana and hemp?
The main difference is that hemp has far less THC than a typical marijuana plant. And unlike THC, CBD is not a psychoactive agent, so theres less possibility that it will cause the same mental confusion, drowsiness or hallucinations that often come with THC.
Is there any truth to the claims that CBD oil can cure cancer?
Right now, no. There is no evidence that CBD oil can cure cancer.
What, if anything, can CBD oil do to alleviate the symptoms of cancer or the side effects of cancer treatment?
Its hard to say if CBD oil can alleviate cancer symptoms or cancer treatmentside effects, because the studies are pretty mixed and even fewer are standardized.
Have any products using CBD-oil been approved by the FDA to treat anything?
What are the dangers of using CBD oil?
Is CBD oil even legal?
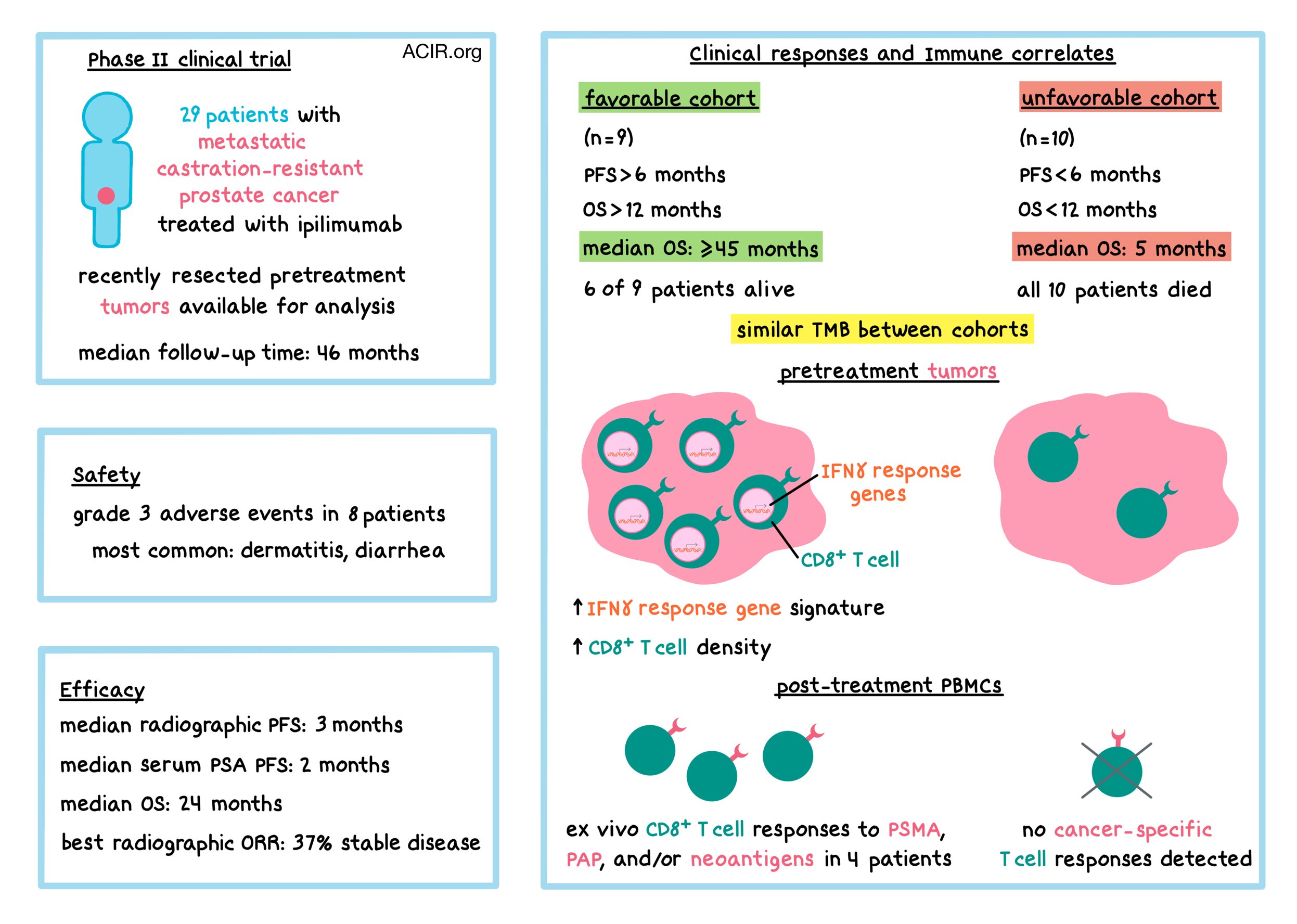
What To Expect With Provenge
Exploratory analysis of IMPACT
In a subanalysis of patients with PSA-matched cohorts, African American men lived longer than Caucasian menc,d
cThe PROCEED registry evaluated the expected safety and survival profile of PROVENGE received by patients in a real-world setting in which there was no control group. The study was conducted to quantify the risk of cerebrovascular events and survival. Patients may have received subsequent anti-cancer interventions per the local investigator’s standard of care.
dThis subgroup analysis of the PROCEED registry was exploratory and results need careful interpretation.
Treatment At Different Stages
The premise that Provenge has a bigger impact when it is used to treat prostate cancer at an earlier stage was investigated through a reanalysis of the original data that led to Provenges initial approval by the FDA. The re-analysis showed that men with the early-stage disease did indeed have a much greater degree of survival prolongation. In fact, the amount of survival prolongation became progressively larger when Provenge was begun sooner.
In this reanalysis, four groups of men, categorized by their different PSA levels at the start of Provenge treatment, were evaluated: men with PSA levels below 22, men with PSA between 22 and 50, men with PSA between 50 and 134, and men with PSA greater than 134.
The table below summarizes the survival of men treated with Provenge, compared with the men treated with placebo, subdivided by the level of PSA at the start of Provenge. The net survival difference between the Provenge and placebo is listed last.
|
PSA Level |
|---|
As the table illustrates, a survival advantage existed for all the Provenge-treated groups compared to the placebo-treated men. However, the amount of survival improvement was greatest in men who began Provenge when PSA was lowest. Men who started Provenge when their PSA was under 22 lived 13 months longer than men at a similar stage who were placebo-treated. Men at very advanced stages, with PSA levels over 134, only lived a few months longer than the men who received a placebo.
Recommended Reading: Does An Enlarged Prostate Affect A Man Sexually
What Does This Mean For Me
If you have advanced colorectal cancer and your tumor is MSI-H/dMMR you may benefit from treatment with the immunotherapy drug Keytruda. This includes newly-diagnosed people who have not yet received treatment for their advanced colorectal cancer.
If you are diagnosed with advanced colorectal cancer, it is important to ask your doctor about tumor testing for microsatellite instability , mismatch repair deficiency or mutations in the BRAF, KRAS and NRAS genes. Knowing your tumor status for these biomarkers can help guide treatment options. In addition, if you are at risk of developing advanced colorectal cancer, talk with your doctor about whether you may be eligible for an ongoing clinical trial.
Lynch syndrome may be linked to MSI-H/dMMR cancers. People diagnosed with colorectal cancer that is MSI-H/dMMR may benefit from genetic counseling and testing to learn if they have Lynch syndrome.
Share your thoughts on this XRAYS article by taking our brief survey.
This article is relevant for:
People with advanced colorectal cancer and a type of biomarker called MSI-High
This article is also relevant for:
People with a genetic mutation linked to cancer risk
Newly diagnosed
People with a family history of cancer
People with metastatic or advanced cancer
Be part of XRAY:
Effectiveness Of Open And Robotic Prostatectomy
Sorry, in progress, not accepting new patients
Prostate cancer is the most common cancer in American men. Surgical removal of the entire prostate is one option among the various ways to treat prostate cancer. The use of robot assistance for prostatectomy has become common place, but its effectiveness has not been compared to standard open prostatectomy in trials carried out at more than one medical institution in which participants are identified and followed forward in time. Robot assisted and standard open prostatectomy health related quality of life outcomes have not been compared in a prospective, multi-centered study. Prostatectomy can have side effects that can change with time. This research study seeks to determine how common and how long-lasting such side effects are to find out what features of individual men’s cancers and what features of the treatments affect those side effects. This study also seeks to identify factors that affect the quality of prostate cancer care by looking at how satisfied men are with their prostate cancer care. Through these findings, this study aims to allow treatment side effects to be anticipated more accurately for individual patients, and to provide a means for determining the quality of prostate care.
San Francisco, California
Read Also: Do Females Have Prostate Cancer
Breast And Lung Cancers
- Non-small cell lung cancer: This is the most common type of cancer that forms in the tissues of the lungs.
- Triple-negative breast cancer: This is a type of breast cancer that does not have any of the common receptors found on breast cancer cells the hormones estrogen and progesterone and a protein called HER2.
Androgen Receptor Directed Therapy On Cognitive Function In Patients Treated With Darolutamide Or Enzalutamide
Sorry, not currently recruiting here
This is a prospective, randomized, open-label phase II study comparing cognitive outcomes between men with non-metastatic and metastatic castration-resistant prostate cancer treated with darolutamide or enzalutamide. Approximately 132 patients will be enrolled. Eligible patients will be randomized in a 1:1 fashion to treatment with enzalutamide 160 mg orally daily or darolutamide 600 mg orally twice daily, in combination with standard LHRH agonist based treatment. Cognitive assessments will be performed using modules from Cambridge Neuropsychological Test Automated Battery an internationally recognized software for assessing cognitive function and impairment.
San Francisco, California
You May Like: Does Enlarged Prostate Cause Constipation
A Trial Looking At Pembrolizumab For Men With Prostate Cancer
Please note – this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Phase:
This trial is for men with prostate cancer that has continued to grow or spread despite hormone treatment.
It is for men who either:
- have had chemotherapy with a drug called docetaxel – this group is now closed
- have had hormone treatment with a drug called enzalutamide. And haven’t had chemotherapy with docetaxel
Apalutamide Plus Cetrelimab In Patients With Treatment
Sorry, not yet accepting patients
Despite the low androgen receptor transcriptional activity of treatment-emergent small cell neuroendocrine prostate cancer, there is persistent AR expression observed in the majority of treatment-emergent small-cell neuroendocrine prostate cancer biopsies. This indicates that epigenetic dysregulation leads to reprogramming away from an AR-driven transcriptional program. Therefore, continuation of AR blockade in the form of apalutamide may provide additive benefit compared to immune checkpoint blockade alone. The investigators hypothesize that the combination of apalutamide plus cetrelimab will achieve a clinically significant composite response rate with sufficient durability of response in mCRPC patients with evidence of treatment-emergent small cell neuroendocrine prostate cancer
San Francisco, California
Also Check: External Prostate Massage Prostatitis
Targeted Radioligand Improves Survival In Advanced Prostate Cancer
Cancer researchers say they have established a new, life-extending treatment option for men with prostate cancer that has spread and become resistant to hormone therapy. The injected treatment combines a targeting compound with a radioactive isotope to irradiate and kill cancer cells.
An international clinical trial sponsored by Endocyte, Inc., a Novartis company tested the targeted radioligand therapy in study participants with advanced prostate cancer. All subjects had cancers that had spread to other organs and continued to progress after previous treatment with two kinds of drugs, androgen axis inhibitors and taxanes. The experimental treatment significantly extended survival, delayed progression and was generally well tolerated by study subjects, researchers said.
This is a completely new treatment option that extends life and disease control in metastatic castration-resistant prostate cancer the most aggressive and deadly type, said Tom Beer, M.D., one of the study leaders and deputy director of the Oregon Health & Science University Knight Cancer Institute.
The added option is particularly important, Beer said, because the existing most effective treatments developed for metastatic castration resistant prostate cancer are now being used to treat early-stage disease.
Some of our best treatments are being used earlier, so by the time you get to metastatic castration resistant disease, you have fewer options, he said.
Finding Prostate Cancer Patients Who Respond To Immunotherapy A Challenge Expert Cautions
byKristin Jenkins, Contributing Writer, MedPage Today December 10, 2019
Pembrolizumab monotherapy demonstrated antitumor activity with manageable safety in a small subgroup of men with previously treated metastatic castrate-resistant prostate cancer , researchers found.
Results from the first three cohorts in the five-cohort phase II, open-label, multi-center KEYNOTE-199 trial showed a combined overall response rate to pembrolizumab monotherapy in nine patients out of 199 in cohorts 1 and 2 combined. The combined median duration of response was 16.8 months, said Johann Sebastian de Bono, MD, PhD, of the Institute of Cancer Research and the Royal Marsden Hospital in London, and colleagues.
An ongoing response was observed in five patients at data cutoff with four responders remaining on treatment after 21.8 months, they reported in the Journal of Clinical Oncology.
“These data add to the growing body of evidence that suggests that, despite its more immunosuppressive microenvironment, a small number of select patients with mCRPC may benefit from pembrolizumab,” the authors wrote.
Patients with DNA repair mutations appeared to respond particularly well to immunotherapy, said de Bono, who added that two of his own patients have been on pembrolizumab for more than 2 years. Local biomarker testing by immunohistochemistry revealed a mismatch repair defect that was below the cutoff for high microsatellite instability by mSINGS assay.
Disclosures
Primary Source
Read Also: Does An Enlarged Prostate Affect A Man Sexually
Safety Pharmacokinetic And Proof
Sorry, in progress, not accepting new patients
The purpose of this study is to assess the safety and activity of ARN-509 in men with advanced castration resistant prostate cancer. Patients will first be enrolled into Phase 1 of the study to identify a tolerable dose for the Phase 2 portion of the study. In the Phase 2, 3 different cohorts of patients will be enrolled to evaluate the safety and activity of ARN-509.
San Francisco, California
What Is Pembrolizumab And Why Can It Be Used For So Many Types Of Cancer
Pembrolizumab is an intravenous medication thats injected into the bloodstream. At this time, its only available as a brand-name product. Specific doses vary by person, but its usually given once every 3 to 6 weeks. People may use it for up to 1 or 2 years.
Pembrolizumab works in a unique way. The researchers that discovered how the immune system works to attack cancer which led to the development of immunotherapy medications like pembrolizumab won the 2018 Nobel Prize in Physiology or Medicine.
Also Check: Flomax Ejaculatory Dysfunction
Hyperpolarized Pyruvate Mr Imaging In Monitoring Patients With Prostate Cancer On Active Surveillance
open to eligible males ages 18 years and up
This phase II trial studies the side how well hyperpolarized carbon C 13 pyruvate magnetic resonance imaging works in monitoring patients with prostate cancer on active surveillance who have not received treatment. Diagnostic procedures, such as MRI, may help visualize HP C-13 pyruvate uptake and breakdown in tumor cells.
San Francisco, California
What Side Effects Are Associated With Immunotherapy Using Pembrolizumab
Like with all other medications, pembrolizumab has a number of potential risks and side effects. Some of the most common side effects of pembrolizumab include:
- Feeling tired
- Pain in muscles, bones, or joints
- Itching
- Fever
- Shortness of breath
Since immunotherapy helps the immune system attack cancer cells, it may also cause the immune system to attack normal cells in the body. These are called immune-mediated adverse events. While rare, pembrolizumab can cause these effects in different parts of the body, including:
- Lungs: Inflammation of the lungs can lead to cough, shortness of breath, or chest pain.
- Intestines: Inflammation of the colon can lead to diarrhea, bloody stools, or severe stomach-area pain.
- Liver: Inflammation of the liver can lead to symptoms like yellowing of the skin or eyes , severe nausea or vomiting, or pain on the right side of the stomach.
- Hormone glands: Pembrolizumab can affect different hormone glands in the body, like the thyroid gland. This may lead to symptoms like headaches that wont go away, a rapid heartbeat, or extreme tiredness. These symptoms can mimic hypothyroidism or hyperthyroidism.
- Kidneys: Inflammation of the kidneys can cause a lesser amount of urine, blood in the urine, or swelling of the ankles.
- Skin: Pembrolizumab can irritate the skin and cause symptoms like rash, itching, or skin blistering.
Read Also: Enlarged Prostate Sexuality
Study Of Pembrolizumab Plus Docetaxel Versus Placebo Plus Docetaxel In Chemotherapy
Sorry, in progress, not accepting new patients
The purpose of this study is to assess the efficacy and safety of the combination of pembrolizumab and docetaxel in the treatment of men with metastatic castration-resistant prostate cancer who have not received chemotherapy for mCRPC but have progressed on or are intolerant to Next Generation Hormonal Agent . There are two primary study hypotheses. Hypothesis 1: The combination of pembrolizumab plus docetaxel plus prednisone is superior to placebo plus docetaxel plus prednisone with respect to Overall Survival . Hypothesis 2: The combination of pembrolizumab plus docetaxel plus prednisone is superior to placebo plus docetaxel plus prednisone with respect to Radiographic Progression-free Survival per Prostate Cancer Working Group -modified Response Evaluation Criteria in Solid Tumors Version 1.1 as assessed by blinded independent central review.
San Francisco, California and other locations
Atezolizumab With Or Without Tocilizumab In Treating Men With Prostate Cancer Before Radical Prostatectomy
open to eligible males ages 18 years and up
This phase II trial studies how well atezolizumab works alone or in combination with tocilizumab in treating men with localized prostate cancer before radical prostatectomy. Immunotherapy with monoclonal antibodies, such as atezolizumab, may help the body’s immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. Androgens can cause the growth of prostate cancer cells. IL-6 is expressed by prostate cancer and within the tumor microenvironment and shown to enhance prostate cancer and disease progression. Treatment with an anti-IL-6 antibody such as tocilizumab may inhibit cancer progression. Giving atezolizumab in combination with tocilizumab may work better in treating prostate cancer.
San Francisco, California and other locations
You May Like: What Happens After Chemo For Prostate Cancer
Another Type Of Immunotherapy
Over the last 30 years, many attempts to harness the immune system have failed. We are beginning to learn that these failures are due to over-activity of the immune systems regulatory component. Whenever the body generates any new immune activity, the activity itself stimulates self-regulation to quell the burgeoning immune response. This is to prevent the development of destructive immune diseases such as lupus, rheumatoid arthritis, or multiple sclerosis.
Now researchers have learned that cancer cells exploit this regulatory component of the immune system by manufacturing immune-suppressive hormones. These hormones lull the immune system to sleep, thus allowing the cancer cells to proliferate by keeping the killer T cells at bay. The regulatory cells, the Treg cells, are in a sense kidnapped and used as a shield to diminish our immune systems anticancer activity. This inability of the immune system to attack cancer is not due to immune weakness rather, it is immune suppression from increased regulatory activity instigated by the cancer cells. With this new understanding, specific pharmaceutical agents have been designed to compensate for this problem.
Initial research evaluating Yervoy in men with prostate cancer shows promise, particularly when combined with radiation . However, more recent studies suggest that another regulatory-blocking medication called Keytruda may work better.
A Phase 1 Study Of Pegilodecakin In Participants With Advanced Solid Tumors
Sorry, in progress, not accepting new patients
This is a first-in-human, open-label, dose escalation study to evaluate the safety and tolerability of pegilodecakin in participants with advanced solid tumors, dosed daily subcutaneously as a monotherapy or in combination with chemotherapy or immunotherapy.
San Francisco, California and other locations
Recommended Reading: What Is The Cpt Code For Mri Prostate
Prostate Cancer ‘super Responders’ Live For 2 Years On Immunotherapy
- Date:
- Institute of Cancer Research
- Summary:
- Some men with advanced prostate cancer who have exhausted all other treatment options could live for two years or more on immunotherapy, a major clinical trial has shown. Researchers found that a small proportion of men were ‘super responders’ and were alive and well even after the trial had ended despite having had a very poor prognosis before treatment.
Some men with advanced prostate cancer who have exhausted all other treatment options could live for two years or more on immunotherapy, a major clinical trial has shown.
Researchers found that a small proportion of men were ‘super responders’ and were alive and well even after the trial had ended despite having had a very poor prognosis before treatment.
The study found that one in 20 men with end-stage prostate cancer responded to the immunotherapy pembrolizumab — but although the number who benefited was small, these patients sometimes gained years of extra life.
The most dramatic responses came in patients whose tumours had mutations in genes involved in repairing DNA, and the researchers are investigating whether this group might especially benefit from immunotherapy.
The phase II clinical trial was led globally by a team at The Institute of Cancer Research, London, and The Royal Marsden Foundation Trust, and involved 258 men with advanced prostate cancer who had previously been treated and become resistant to androgen deprivation therapy and docetaxel chemotherapy.
