Urine Test For Prostate Problems
The GP may refer you to a urologist or other appropriate specialist if:
- previous treatments have not helped your urinary problems
- a urinary infection does not go away or comes back regularly
- you cannot fully empty your bladder
- you have kidney problems
- you have stress incontinence, which is when urine leaks out at times when your bladder is under pressure for example, when you cough or laugh
You should also see a specialist if the GP is concerned that your symptoms could be caused by cancer, although for most men this is not the cause.
To help find out what might be causing your symptoms and decide how to manage them, you should be offered extra tests to measure:
- how fast your urine flows
- how much urine is left in your bladder after you have peed
You may also be offered other tests, depending on your symptoms or the treatment you and your doctor are considering.
Page last reviewed: 10 February 2020 Next review due: 10 February 2023
The Prostate Cancer Test
Considering prostate cancers prevalence in society it is the most common cancer in men in the UK and with the knowledge of enhanced biomarker prevalence early in the day, Clark set out to develop a test that could effectively process samples collected at home, early in the morning, without the need for patients to travel to provide the sample.
The resultant prostate urine risk test also circumvents the need for more invasive, time consuming and uncomfortable diagnostic methods for prostate cancer diagnosis, such as blood tests, biopsy, MRI and digital rectal examination .
The PUR test measures gene expression in the urine samples, which can be posted to labs for analysis, and can provide key indicators of the malignancy of a tumor.
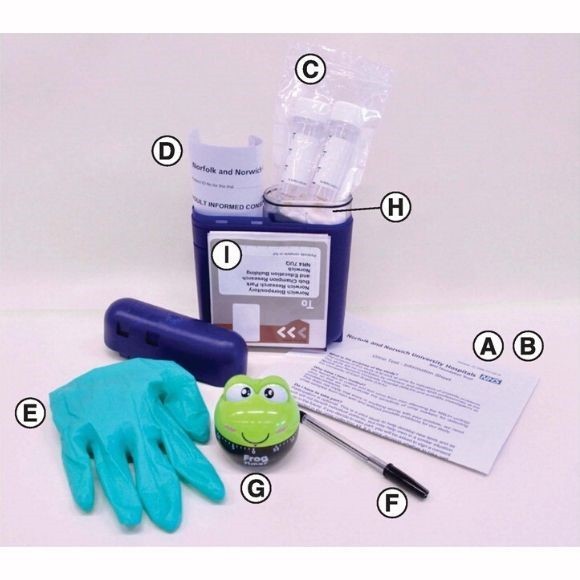
Contents of the at-home collection kit. Invitation to participate. Study information sheet. Two urine collection tubes containing dried Norgen preservative. Two consent forms . Disposable non-allergenic glove. Pen . 1-h frog timer. Sealable plastic bag with wadding. Preaddressed postage-paid SafeBox for returning the samples.
Prostate Tissue Specimen Cohort
The GSE17951 prostate tissue specimen cohort includes quantitative mRNA expression data of PCa and benign prostate specimens obtained from Affymetrix U133Plus2 array . The PCa tissues in the cohort were collected from patient biopsy specimens, and the benign prostate tissues were obtained from prostate autopsy specimens of patients with benign disease. The gene expression levels of the 25 genes in the panel were obtained from the database and normalized with beta-actin expression level.
Don’t Miss: Can Prostate Cancer Spread To The Testicles
Your Psa Is Going Up Whats Going On With Your Prostate Good News: Second
Your PSA is going up. Whats going on with your prostate? Do you need a biopsy? Or, maybe youve already had a biopsy that didnt find cancer, but your urologist is wondering whether you need another one. Whats the next step?
Good news: You dont have to move directly to having needles stuck in your prostate! Its not the Monopoly bad-case-scenario of Do not pass Go, do not collect $200! There is a next step! Its a second-line test: a blood or urine test that can provide other layers of information beyond the basic PSA test. There are several good ones out there. Which one do you need? Well, as Marlon Brando said in the classic 1953 movie, The Wild One: Whadya got?
Theres no shortage of options! There are blood tests that provide more nuanced information than the basic PSA test, plus urine tests and even, if youve already had a biopsy, molecular biomarker tests, which arent done on body fluids but on tissue samples. These tests can be helpful, not only in diagnosing cancer, but in risk stratification predicting which cancer is more likely to be aggressive, and which cancer is less likely to need immediate treatment.
Now, about those other blood tests: In addition to the free PSA test, here are two more that include free and total PSA, but look for other factors, as well:
What Is The Prostate

The prostate is a walnut-shaped gland that is part of the male reproductive system. It has two or more lobes, or sections, enclosed by an outer layer of tissue. The prostate is located in front of the rectum and just below the bladder, where urine is stored. It surrounds the urethra at the neck of the bladder and supplies fluid that goes into semen.
Also Check: Green Tea Prostate Cancer Mayo Clinic
You May Like: How To Check If My Prostate Is Enlarged
Future Prostate Cancer Biomarkers
The use of advanced technologies such as NGS and machine learning have led to a wide array of prostate cancer urine biomarkers. Currently, multiplex panels in development combine classes of biomarkers such as proteins and nucleic acids and utilize a broader spectrum of known mutational anomalies and available biomarkers. Challenges remain to validate and normalize the numerous available biomarkers while improving sensitivity and specificity.
Urine Test Could Help Prevent Cervical Cancer: Study
Prostate cancer usually develops slowly and the majority of cancers will not require treatment in a mans lifetime. However, doctors struggle to predict which tumours will become aggressive, making it hard to decide on treatment for many men, said lead researcher Dr Jeremy Clark from UEAs Norwich Medical School.
The most commonly used tests for prostate cancer include blood tests, a physical examination known as a digital rectal examination , an MRI scan or a biopsy.
We developed the PUR test, which looks at gene expression in urine samples and provides vital information about whether a cancer is aggressive or low risk.
The research team provided 14 participants with an At Home Collection Kit, and instructions.
They then compared the results of their home urine samples, taken first thing in the morning, with samples collected after a digital rectal examination.
We found that the urine samples taken at home showed the biomarkers for prostate cancer much more clearly than after a rectal examination. And feedback from the participants showed that the at home test was preferable, the authors wrote in a paper published in the journal BioTechniques.
Because the prostate is constantly secreting, the collection of urine from mens first urination of the day means that the biomarker levels from the prostate are much higher and more consistent, so this is a great improvement, they added.
Read Also: How Long Can You Live With Prostate Cancer Without Treatment
More Clinically Accurate Biomarkers Could Help Prevent Unnecessary Prostate Biopsies
Prostate cancer is the most common non-cutaneous malignancy in men. Traditionally, prostate specific antigen is used to screen for disease, whereas a prostate biopsy is required for diagnosis. However, the specificity for PSA is only 2045 percent,1 leading to a high number of unnecessary prostate biopsies. Many low-risk prostate cancers are suitable for surveillance alone, so there is an urgent need for minimally invasive prostate cancer biomarkers to accurately distinguish low-risk from aggressive disease.
Urine biomarkers are an attractive option as prostate cancer cells are shed into urine, making screening minimally invasive. Urine biomarkers also assess the entirety of the prostate, unlike prostate biopsies, which represent a small fraction of the cancer. Accurate urinary biomarkers further minimize unnecessary prostate biopsies while accurately determining which prostate cancers require aggressive treatment.
Retrospective And Prospective Urine Cohorts
A multicenter retrospective study was conducted at San Francisco General Hospital with Institutional Review Board approval to collect and test archived urine sediments to identify and validate urine biomarkers for PCa diagnosis and prognosis. The prospectively designed, retrospective study used pre-biopsy urine samples randomly chosen from sample archives at the Cooperative Human Tissue Network Southern Division and Indivumed GmbH . This study followed the REMARK guidelines. With prior ethical approval and patient consent for future studies, urine samples were collected from 520 patients who had elevated PSA or symptoms and were diagnosed to have prostate cancer by routine biopsy after the urine collection. The patients were recruited from July 2004 to November 2014 with follow-up through June, 2015.
During the follow-up period, all the patients who had radical prostatectomy or other treatments were assessed periodically for biochemical recurrence and cancer metastasis .
Don’t Miss: Does Prostate Cancer Cause Itching
Urine Test For Prostate Cancer
Synopsis:The research surrounding the new urine test is designed to determine its ability to stratify men diagnosed with prostate cancer. Researchers at the University of Michigan Health System are exploring a new urine test designed to identify two genetic markers in men. These bio-markers, TMPRSS2:EG and PCA3, are known to be present in prostate cancer patients. More than one million prostate biopsies are performed annually in the Unites States, most often as a follow-up to the detection of elevated PSA levels. Like other medical tests, PSA screenings can produce false results, so it is possible that some biopsies are performed on men who don’t actually have raised PSA levels.
Whats The Difference Between The Pca3 Test And The Psa Test
The PCA3 test measures the levels of prostate cancer gene 3. This gene is found in high levels in prostate cancer cells. The test isnt affected by an enlarged prostate, prostatitis, or other conditions of the prostate gland.
PSA tests measure the levels of prostate-specific antigen in your blood. If your levels are high, or if they rise rapidly, you may have prostate cancer. But elevated PSA levels can be caused by many things besides prostate cancer, including:
- benign prostatic hyperplasia , often referred to as an enlarged prostate, which is a common, noncancerous condition
- prostatitis, or inflammation or infection of the prostate
- pressure on the prostate from a digital rectal exam or catheter
- ejaculation
PSA tests used to be given annually to men older than 50 years, but theyre no longer recommended as a primary screening method by most medical experts. There are several reasons for this:
- There are a high number of false positives with PSA tests.
- Some men have prostate cancer even when their PSA levels are low, so the test may give false negatives.
- In many men, prostate cancer grows so slowly that watchful waiting is advised rather than treatment.
- Because the diagnosis of cancer can be alarming, some men have unnecessary biopsies or surgery.
- Incontinence and sexual problems can be common side effects of prostate cancer treatment.
Don’t Miss: Can You Get Prostate Cancer Without A Prostate
Urine Sample Processing And Gene Expression Quantification
The procedures for urine sample processing and gene expression quantification were performed as described . In the retrospective study, 1015 ml urine samples were collected without prior digital rectal examination and the urine pellet was flash-frozen and stored at 80°C. In the prospective study, 1545 ml urine was collected without prior DRE and the urine was stored with 5 ml DNA/RNA preservative AssayAssure or U-Preserve at 4°C and processed within a week. The urine was centrifuged at 1,000 × g for 10 min. The pellet was washed with phosphate-buffered saline and centrifuged again at 1,000 × g for 10 min. The cell pellet was then used for RNA purification or frozen immediately on dry ice followed by storage at 80°C. The procedures for gene expression quantification were performed as described and the details are provided in the Supplementary Data.
Data Analysis And Algorithm For Identification Of Clinically Significant Prostate Cancer
Gene expression data were downloaded and analyzed using ABI Quantstudio 6 software . The mean cycle threshold value from triplicate PCR was used as the gene expression level of each gene . The housekeeping gene beta-actin was measured and used to normalize each gene in the classifier .
For the identification of clinically significant and insignificant PCa by the 24-Gene Classifier in the urine samples, CtS of the 24 genes was used in the following Urine Clinically Significant Cancer Algorithm:
CUrineCSC = AH+
* 24 are gene 1 and gene 1 cross clinically insignificant PCa regression coefficients through gene 24 and gene 24 cross clinically insignificant PCa regression coefficients. The sample was diagnosed as clinically significant PCa when the Urine Clinically Significant Cancer D Score was > 0, whereas the sample was diagnosed as clinically insignificant PCa when the D Score was 0.
The diagnostic method of clinically significant and insignificant PCa by the 24-Gene Classifier in the prostate tissue specimens is described in the Supplementary Data.
Don’t Miss: How To Self Milk Prostate
Development And Validation Of A 24
We recently developed an improved method to detect mRNA expression of biomarker genes by cDNA pre-amplification before real-time qRT-PCR using urine samples collected without digital rectal examination . The method is robust and can be used for biomarker classifiers as non-invasive and convenient urine tests . We tested if the 24-Gene Classifier could detect clinically significant and insignificant cancer in cell pellets of the urine samples collected without DRE using the same method. We conducted two independent, multicenter retrospective and prospective studies to collect pre-biopsy urine samples. The patients in both cohorts were real patients from participating hospitals. The patient characteristics and clinicopathological parameters are shown in Table 1. The study endpoint was to measure the diagnostic performance of the 24-Gene Classifier urine test for the diagnosis of clinically significant and insignificant cancer after PCa diagnosis to determine if the patient needs treatment or active surveillance .
Table 3. Diagnostic performance of the 24-Gene Classifier urine test for identification of clinically significant prostate cancer by discriminant analysis in a retrospective cohort , a prospective cohort , and a combination cohort .
The 24-Gene Classifier with the algorithm was validated in an independent prospective cohort and showed a sensitivity of 86.0% , specificity of 97.7% , and AUC of 0.959 .
What Happens During The Test
Your doctor will start by giving you a digital rectal exam . They will insert a lubricated, gloved finger in your rectum and gently push on your prostate gland. This helps move the PCA3 into your urethra so it can be expelled in your urine. Following the DRE, youll be asked to provide a urine sample. The urine sample will be sent to a lab for testing and the results will then be sent to your doctor when they are available.
The PCA3 test results are more accurate when preceded by a DRE.
Also Check: What Does Stage 7 Prostate Cancer Mean
New Prostate Cancer Urine Test Shows How Aggressive Disease Is And Could Reduce Invasive Biopsies
Published by Communications
Researchers from the University of East Anglia have developed a new urine test for prostate cancer which also shows how aggressive the disease is.
A new study published today shows how an experimental new test called ExoGrail has the potential to revolutionise how patients with suspected prostate cancer are risk-assessed prior to an invasive biopsy.
The research team say their new test could reduce the number of unnecessary prostate cancer biopsies by 35 per cent.
Prostate cancer is the most common cancer in men in the UK. It usually develops slowly and the majority of cancers will not require treatment in a mans lifetime.
The most commonly used tests for prostate cancer include blood tests, a physical examination known as a digital rectal examination , an MRI scan or an invasive biopsy.
However, doctors struggle to predict which tumours will become aggressive, making it hard to decide on treatment for many men.
Lead researcher Dr Dan Brewer, from UEAs Norwich Medical School and also a visiting worker at the Earlham Institute, said: While prostate cancer is responsible for a large proportion of all male cancer deaths, it is more commonly a disease men die with rather than from.
Invasive biopsies come at considerable economic, psychological and societal cost to patients and healthcare systems alike.
The ExoGrail test also provided risk scores for patients and highlighted those for which an invasive biopsy would have been beneficial.
Liquid Sample Preparation And Cell
Peripheral blood was collected in EDTA vacutainer tubes and processed within 2 hours. The blood samples were allowed to clot for 30 min before centrifugation for 15 minutes at 1000 x g and the plasma was collected and stored at -80°C prior to cfDNA extraction. For urine sample collection, an in-house urine collection kit was developed to maintain the integrity of urinary cfDNA and to facilitate the transportation of urine samples. Morning urine was obtained through the urine collection cup and transferred into four vacuum tubes, where the urine samples were mixed thoroughly with prefilled preservation buffer.
Read Also: Can Prostate Biopsy Cause Ed
New Prostate Cancer Tests Using Urine Samples
The urine samples give information not only on gene fragments of prostate cancer but also on the risk factors. The University of Michigan developed another urine-based test that can reduce the number of negative biopsies by 50%.
This same new test was found to be 97% accurate in identifying cancers later found to be aggressive in biopsies. This means that the urine test can identify prostate cancer earlier than other tests and also divide people into different risk groups, allowing the doctor to accurately determine the treatment path watchful waiting, active surveillance, biopsy or immediate treatment.
In the following video, Dr. David Samadi discusses the benefits of this new prostate cancer test:
Selectmdx Liquid Biopsy Has Been Added To The Most Recent 2018 Update Of The Guidelines Of The European Association Of Urology In The Diagnostic Workup Of The Men Being Considered For Prostate Biopsy
If you are being considered for your primary or secondary prostate biopsy due to an elevated PSA test result you might want to consider the SelectMDx Liquid Biopsy a noninvasive diagnostic laboratory test performed on a urine sample which measures the likelihood of discovering prostate cancer during a prostate biopsy procedure. The test measures mRNA levels of DLX1 and HOXC6 . The test has been thoroughly evaluated during clinical trials*.
SelectMDx Liquid Biopsy allows the Patient and their physician to undertake an informed decision regarding the necessity of performing primary or secondary prostate biopsy. Thus the test potentially enables the Patient to avoid any further, unnecessary, invasive diagnostic procedures and their potential complications .
HIFU CLINIC Prostate Cancer Center is the first clinic in Poland and one of the first clinics in Europe to offer this breakthrough test for its Patients.
Read Also: Can An Enlarged Prostate Cause Erectile Problems
