Enzalutamide With Or Without Abiraterone And Prednisone In Treating Patients With Castration
Sorry, in progress, not accepting new patients
This randomized phase III trial studies enzalutamide to see how well it works compared to enzalutamide, abiraterone, and prednisone in treating patients with castration-resistant metastatic prostate cancer. Androgens can cause the growth of prostate cancer cells. Drugs, such as enzalutamide, abiraterone acetate, and prednisone, may lessen the amount of androgens made by the body.
San Francisco, California
Atezolizumab With Or Without Tocilizumab In Treating Men With Prostate Cancer Before Radical Prostatectomy
open to eligible males ages 18 years and up
This phase II trial studies how well atezolizumab works alone or in combination with tocilizumab in treating men with localized prostate cancer before radical prostatectomy. Immunotherapy with monoclonal antibodies, such as atezolizumab, may help the bodys immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. Androgens can cause the growth of prostate cancer cells. IL-6 is expressed by prostate cancer and within the tumor microenvironment and shown to enhance prostate cancer and disease progression. Treatment with an anti-IL-6 antibody such as tocilizumab may inhibit cancer progression. Giving atezolizumab in combination with tocilizumab may work better in treating prostate cancer.
San Francisco, California and other locations
You May Like: What Happens After Chemo For Prostate Cancer
Pet Imaging Study Of 89zr
open to eligible people ages 18 years and up
CD46 is an exciting new therapeutic target in prostate cancer, with the antibody drug conjugate FOR46 under investigation in phase I clinical trials. The hypothesis of the study is that CD46 expression, measured via our novel imaging biomarker, is a characteristic feature of mCRPC, and particularly common in the most lethal forms of the disease including adenocarcinoma and Small-cell neuroendocrine carcinoma . These data will provide crucial information about the feasibility of targeting cluster of differentiation 46 in mCRPC, will be used guide the development of novel therapeutic and theranostic agents, to help develop treatments that improve outcomes for men with the most lethal forms of prostate cancer.
San Francisco, California
You May Like: Does Dht Cause Prostate Cancer
What Does Keytruda Cost
Costs of prescription drugs can vary depending on many factors. These factors include what your insurance plan covers and which pharmacy you use. To find current prices for Keytruda in your area, visit WellRx.com.
If you have questions about how to pay for your prescription, talk with your doctor or pharmacist. You can also visit the Keytruda manufacturers website to see if it has support options.
Immunotherapies Have Come Of Age
The development this past decade of cancer immunotherapies has seen several triumphs like Keytruda.
Drugs that harness the bodys immune system to fight cancer have begun to change the paradigm of cancer treatment.
But its still a relatively new field. There have been some bumps along the road.
CAR T-cell immunotherapy, for example, is a promising treatment. In some cases, it can even cure certain blood cancers.
However, there have been some with CAR-T, from cytokine release syndrome to neurotoxicity.
But the safety profile of each subsequent generation of CAR-T appears to improve on the last.
You May Like: Does Prostatitis Cause Abdominal Pain
Scheduling Treatments With Keytruda
- In adults, KEYTRUDA is usually given every 3 weeks or every 6 weeks depending on the dose that you are receiving. In children, KEYTRUDA is usually given every 3 weeks.
- Talk to your doctor about the treatment schedule that is right for you. Your doctor can help answer questions.
30 minutes per intravenous infusion
The adult dose given every 6 weeks is approved based on specific types of data showing how this dose works in the body. Studies are ongoing to provide additional information about clinical benefit.
Merck’s Keytruda Takes Another Hit With Phase Iii Prostate Cancer Fail
Merck‘s Keytruda has taken another hit after the drug failed to meet dual primary endpoints in the Phase III KEYNOTE-921 trial for metastatic castration-resistant prostate cancer .
The randomized, double-blind study enrolled 1,030 patients who received either 200mg of Keytruda every three weeks for around two years plus prednisone and chemotherapy or a placebo with prednisone and chemotherapy. The participants were mCRPC patients who have not undergone chemotherapy but whose disease has progressed or are intolerant to a next-generation hormonal agent.
The two primary endpoints were overall survival and radiographic progression-free survival . The secondary endpoints were prostate-specific antigen response rate, time to initiation of the first subsequent anti-cancer therapy, duration of response and objective response rate.
At the end of the study, although no new safety concerns were observed, Keytruda did not demonstrate significant improvements based on the pre-specified statistical plan. Full details will be shared at a future medical conference.
Keytruda is an anti-programmed death receptor-1 therapy that works by boosting the immune system’s ability to detect and kill tumor cells. Prostate cancer is the second most common cancer in men worldwide, and 10% to 20% of patients with the advanced version are likely to develop CRPC in five years. At least 85% of these will have metastases upon CRPC diagnosis.
Recommended Reading: How Does One Get Prostate Cancer
What Is Pembrolizumab And Why Can It Be Used For So Many Types Of Cancer
Pembrolizumab is an intravenous medication thats injected into the bloodstream. At this time, its only available as a brand-name product. Specific doses vary by person, but its usually given once every 3 to 6 weeks. People may use it for up to 1 or 2 years.
Pembrolizumab works in a unique way. The researchers that discovered how the immune system works to attack cancer which led to the development of immunotherapy medications like pembrolizumab won the 2018 Nobel Prize in Physiology or Medicine.
Gb1275 Monotherapy And In Combination With An Anti
Sorry, in progress, not accepting new patients
This first-in-human study is an open-label, multicenter study that consists of a Phase 1 Dose Escalation/Expansion phase of GB1275 monotherapy or in combination with Anti-PD-1 Antibody or in combination with Standard of Care in Patients with Metastatic Pancreatic Adenocarcinoma followed by a Phase 2 Basket Expansion phase in Patients with Specified Metastatic Solid Tumors
San Francisco, California and other locations
Don’t Miss: What Does Prostate Fluid Look Like
How To Take Keytruda
Keytruda is given through the veins every three to six weeks for as long as your oncologist recommends. It will usually take 30 minutes for each infusion.
The medication will be administered in a hospital or medical facility under the supervision of healthcare providers. Keep up with your appointments, and don’t hesitate to discuss any concerns with the healthcare team.
> > > 1 Bedtime Hack To Pee Like A Bull
An enlarged prostate can also be the cause of other problems. If the enlarged prostate is causing symptoms, the best treatment would be a natural remedy. In the meantime, there are treatments for a wide range of conditions that cause a man to experience pain. A common surgical procedure involves an electric loop, laser, or electro-stimulation. The procedure is a safe and effective option for treating enlarged or symptomatic BPH.
Read Also: Can You Live Without A Prostate
Promote: Identifying Predictive Markers Of Response For Genitourinary Cancer
Sorry, in progress, not accepting new patients
This is a tissue and blood collection protocol requiring image-guided biopsies of metastatic prostate cancer and other genitourinary malignancies including renal cell carcinoma and urothelial carcinoma. Whenever possible, a new bone lesion or new/progressing soft tissue lesion will be chosen for biopsy as opposed to radiographically stable lesion. Patients will be enrolled in into one of several parallel cohorts based upon disease status or type and the planned systemic therapy following baseline tumor biopsy: Androgen signaling inhibition, Immunotherapy, Radiotherapy, Targeted Therapy/Investigational therapeutic, DNA damage response pathway, Aggressive variant disease, Castration-sensitive ADT naïve and ADT < 3 months), or Castration-sensitive pre-treated with sub-optimal PSA nadir > 0.2 ng/ml, metastatic renal cell carcinoma and metastatic and urothelial carcinoma.
San Francisco, California
To Evaluate If Green Tea Can Be Effective In Reducing The Progression Of Prostate Cancer In Men On Close Monitoring

open to eligible people ages 21 years and up
This phase II trial studies how well green tea catechins work in preventing progression of prostate cancer from a low risk stage to higher risk stages in men who are on active surveillance. Green tea catechins may stabilize prostate cancer and lower the chance of prostate growing.
San Mateo, California
Read Also: New Prostate Cancer Treatment 2021
Keytruda Benefits A Small Proportion Of Men With Late
HomeNews & MediaNewsKeytruda benefits a small proportion of men with late-stage metastatic prostate cancer
10 December 2019
.
Keytruda is an immunotherapy drug that has revolutionised treatment for cancers such as melanoma and lung cancer. Unfortunately, the same level of success has not been seen for prostate cancer. Now a new study has some hopeful results, showing a small proportion of men with late-stage prostate cancer will benefit from Keytruda.
In May 2017, the US Food and Drug Administration for the first time approved a cancer treatment based on a common biomarker, rather than the location in the body where the tumour originated. The drug, Keytruda , was approved for use by patients who have tumours carrying a genetic defect that makes them susceptible to the drug. Keytruda is a very successful cancer drug that is now used to treat many different types of cancer in Australia.
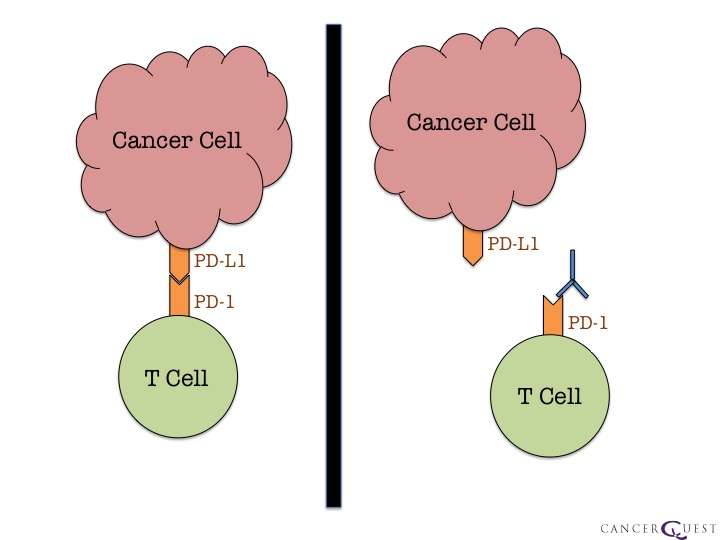
How does Keytruda work?
Keytruda is called a checkpoint blockade inhibitor. It targets proteins called PD-1 receptors on the surface of immune cells. This receptor is responsible for stopping the immune system from fighting against the bodys own cells. Tumours make inhibitors that bind to this receptor, shutting down all immune responses from the cells with PD-1 receptors. By stopping this happening, Keytruda makes it easier for the immune cells to recognise cancer cells and fight against them.
The Keynote-199 study
Cohort 2 : tumour samples did not show inhibitors of PD-1.
What Side Effects Are Associated With Immunotherapy Using Pembrolizumab
Like with all other medications, pembrolizumab has a number of potential risks and side effects. Some of the most common side effects of pembrolizumab include:
- Feeling tired
- Fever
- Shortness of breath
Since immunotherapy helps the immune system attack cancer cells, it may also cause the immune system to attack normal cells in the body. These are called immune-mediated adverse events. While rare, pembrolizumab can cause these effects in different parts of the body, including:
- Lungs: Inflammation of the lungs can lead to cough, shortness of breath, or chest pain.
- Intestines: Inflammation of the colon can lead to diarrhea, bloody stools, or severe stomach-area pain.
- Liver: Inflammation of the liver can lead to symptoms like yellowing of the skin or eyes , severe nausea or vomiting, or pain on the right side of the stomach.
- Hormone glands: Pembrolizumab can affect different hormone glands in the body, like the thyroid gland. This may lead to symptoms like headaches that wont go away, a rapid heartbeat, or extreme tiredness. These symptoms can mimic hypothyroidism or hyperthyroidism.
- Kidneys: Inflammation of the kidneys can cause a lesser amount of urine, blood in the urine, or swelling of the ankles.
- Skin: Pembrolizumab can irritate the skin and cause symptoms like rash, itching, or skin blistering.
You May Like: What Are The Risks Of Having A Prostate Biopsy
Breast And Lung Cancers
- Non-small cell lung cancer: This is the most common type of cancer that forms in the tissues of the lungs.
- Triple-negative breast cancer: This is a type of breast cancer that does not have any of the common receptors found on breast cancer cells the hormones estrogen and progesterone and a protein called HER2.
When Will I Need To Stop Using Keytruda
Your doctor may have you stop Keytruda treatment early if:
- your cancer isnt responding well to Keytruda, or
- youre having bothersome or severe side effects from the drug
But, even if your cancer remains stable and youre tolerating Keytrudas side effects, your doctor may have you stop treatment after a certain amount of time. This is because the long-term effects of Keytruda arent known.
In studies, the length of Keytruda treatment was limited to about 2 to 3 years. But this depended on the type of cancer being treated.
Talk with your doctor to find out how long you might need to take Keytruda.
You May Like: Do You Have To Have A Prostate
A Parallel Arm Phase 1b/2a Study Of Dkn
Sorry, in progress, not accepting new patients
This is a non-randomized multi-center Phase 1b/2a dose escalation and dose expansion study involving 85-97 patients testing DKN-01 as monotherapy or in combination with docetaxel in metastatic castration-resistant prostate cancer. Patients need to be biomarker positive either in plasma or biopsy. Other biopsies for correlative studies are encouraged but not mandatory. Pharmacokinetic testing of one pre-treatment blood sample and one post-treatment blood sample will be mandatory on Day 1 of every cycle.
San Francisco, California
Gemcitabine Hydrochloride And Cisplatin With Or Without Bevacizumab In Treating Patients With Advanced Urinary Tract Cancer
Sorry, in progress, not accepting new patients
This randomized phase III trial studies gemcitabine hydrochloride, cisplatin, and bevacizumab to see how well they work compared with gemcitabine hydrochloride and cisplatin in treating patients with urinary tract cancer that has spread to other places in the body. Drugs used in chemotherapy, such as gemcitabine hydrochloride and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Immunotherapy with bevacizumab, may induce changes in body’s immune system and may interfere with the ability of tumor cells to grow and spread. It is not yet known whether gemcitabine hydrochloride and cisplatin are more effective when given with or without bevacizumab in treating patients with urinary tract cancer.
San Francisco, California
Read Also: When To Get Prostate Test
Merck Provides Update On Phase 3 Keynote
RAHWAY, N.J.—-Merck , known as MSD outside the United States and Canada, today announced that the Phase 3 KEYNOTE-921 trial evaluating KEYTRUDA in combination with chemotherapy compared to chemotherapy alone did not meet its dual primary endpoints of overall survival and radiographic progression-free survival for the treatment of patients with metastatic castration-resistant prostate cancer . In the study, there were modest trends toward an improvement in both OS and rPFS for patients who received KEYTRUDA plus chemotherapy compared with chemotherapy alone however, these results did not meet statistical significance per the pre-specified statistical plan. The safety profile of KEYTRUDA in this trial was consistent with that observed in previously reported studies. Results will be presented at an upcoming medical meeting.
Results from this study serve as an important reminder that metastatic prostate cancer remains very difficult to treat, and more research is needed. We will continue to advance our clinical development program to evaluate KEYTRUDA-based combinations and novel candidates for patients with this disease, said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories. We are grateful to the patients and investigators for their participation in this study.
About KEYNOTE-921
About metastatic castration-resistant prostate cancer
About KEYTRUDA® injection, 100 mg
Melanoma
Lactation
A Study Of Xmab20717 In Subjects With Selected Advanced Solid Tumors
Sorry, in progress, not accepting new patients
This is a Phase 1, multiple dose, ascending dose escalation study to define a MTD/RD and regimen of XmAb20717, to describe safety and tolerability, to assess PK and immunogenicity, and to preliminarily assess anti-tumor activity of XmAb20717 in subjects with selected advanced solid tumors.
San Francisco, California
Also Check: Late Stage Prostate Cancer Treatment
Is Keytruda Used For Prostate Or Pancreatic Cancer
No, Keytruda isnt currently used for prostate cancer or pancreatic cancer.
One study showed that Keytruda may be effective and safe for a specific type of prostate cancer called programmed death ligand 1 -positive, metastaticcastration-resistant prostate cancer.
But more studies are still needed to look at treating prostate cancer with Keytruda.
For pancreatic cancer, recent studies have looked at using immunotherapy as treatment. Researchers are currently a drug combination that includes pembrolizumab for advanced pancreatic cancer.
Talk with your doctor if youre interested in treatment options for prostate cancer or pancreatic cancer.
Keytruda Fluffs Its Lines In Prostate Liver Cancer Trials
Two phase 3 trials of Merck & Cos Keytruda in metastatic castration-resistant prostate cancer and advanced liver cancer have ended in failure, proving once again that cancer immunotherapy studies can be a hit-and-miss affair.
The CRPC trial KEYNOTE-921 looked at the combination of Keytruda with docetaxel in more than 1,000 patients who had not been treated with chemotherapy before, but who had seen disease progression despite earlier treatment with an anti-hormonal therapy.
The combination was compared to placebo plus docetaxel in the study, but was unable to show a significant improvement in overall survival or radiographic progression-free survival the trials two primary endpoints.
Merck said there were modest trends towards improvement with Keytruda, but overall the study was a failure, reflecting that metastatic CRPC is very difficult to treat.
It unfortunately ties in with a prevailing trend of late-stage trial failures for checkpoint inhibitors in CRPC, including the recently-reported KEYLYNK-010 trial of Keytruda and Merck/AstraZenecas PARP inhibitor Lynparza in CRPC patients previously treated with hormonal therapy and chemo.
Keytruda has struggled to make an impact in prostate cancer, and the disease does not feature on the drugs very long list of approved indications, despite considerable clinical testing on Mercks part, or indeed on the label of other drugs in the PD-1/PD-L1 inhibitor class like Bristol-Myers Squibbs Opdivo .
Recommended Reading: What Is The Procedure For Prostate Surgery
Leveraging Technology To Achieve Equity For Men With Prostate Cancer On Androgen Deprivation Therapy
open to eligible males ages 18 years and up
This clinical trial studies a digital platform, the supportive therapy in androgen deprivation , in achieving equity for men undergoing treatment with androgen deprivation therapy for prostate cancer. STAND-T is a digital platform that provides prostate health information, evidence-based materials and resources. STAND-T may help improve health, address symptoms, and promote equity in men with prostate cancer.
San Francisco, California
